Effectiveness and Persistency of Ustekinumab Treatment for Ulcerative Colitis: A Phoenix retrospective Cohort Study
- PMID: 38711858
- PMCID: PMC11071518
- DOI: 10.1093/crocol/otae024
Effectiveness and Persistency of Ustekinumab Treatment for Ulcerative Colitis: A Phoenix retrospective Cohort Study
Abstract
Background: Real-world data regarding ustekinumab (UST) for ulcerative colitis (UC) particularly in biologics-naïve patients is currently limited. This study aimed to elucidate the real-world effectiveness and safety of UST for UC.
Methods: Overall, 150 patients with UC treated with UST from March 2020 to January 2023 were enrolled across 7 referral hospitals. To assess the clinical efficacy and persistence of UST, retrospective analyses were conducted from weeks 8 to 56. Predictive factors concerning the response and persistence of UST were examined through univariate and multivariate analyses.
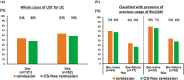
Results: Of the 150 patients, 125 received UST for remission induction, including 36% biologics-naïve. The response and remission rates were 72.8% and 56.0% at week 8 and 73.2% and 63.4% at week 56, respectively. Biologics-naïve patients represented higher response and remission rates at week 8 (84.4% and 73.3%) than those with biologics exposure (66.2% and 46.2%). Patients with prior antitumor necrosis factor (anti-TNF) and vedolizumab (VDZ) exposure had relatively lower response and remission rates (34.5% and 24.1%, respectively). The 1-year cumulative persistence rate was 84.0%. Multivariate analysis revealed that the chronic continuous type and prior anti-TNF and VDZ exposure were negative predictive factors for week 8 responsiveness. Clinical response at week 8 was a predictor of 1-year persistence. Adverse event incidence remained notably low at 6.4%.
Conclusions: This study highlights the safety and effectiveness of UST as an induction and maintenance therapy for UC. Chronic continuous type and previous anti-TNF and VDZ exposure negatively contributed to short-term effectiveness, whereas short-term effectiveness provided good persistency.
Keywords: bio-failure; bio-naïve; persistency; ulcerative colitis; ustekinumab.
© The Author(s) 2024. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
K.A. received lecture fees from Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, AbbVie GK., Pfizer Inc., JIMRO Co. Ltd., EA Pharma Co. Ltd., Mochida Pharmaceutical Co. Ltd. M.F. has received honoraria and had expenses paid to attend or give a presentation or advice at a meeting for EA Pharma Co., Ltd., AYUMI Pharmaceutical Corporation, AbbVie GK, Otsuka Pharmaceutical Factory, Inc., Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Nippon Kayaku Co., Ltd., elpharma Co., Ltd., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Olympus Corporation, Celltrionhealthcare.jp, Alfresa Pharma Corporation, Mylan Inc., Boston Scientific Corporation, Covidien Japan, Inc., FUJIFILM Corporation, Fuji Chemical Industries Co., Ltd., JIMRO Co., Ltd. and received research grants from EA Pharma Co., Ltd., AYUMI Pharmaceutical Corporation, AbbVie GK, Otsuka Pharmaceutical Factory, Inc., Zeria Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nobelpharma Co., Ltd., Pfizer Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Limited, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., JIMRO Co., Ltd., Kamui Pharma. Inc. N. U. has received grant from Pfizer Inc. T.I. received lecture fees from Janssen Pharmaceutical Co., Ltd. and Grants for commissioned/joint research from Gilead Sciences, Inc., Janssen Pharmaceutical K.K., Eli lilly Co., Ltd., Takeda Pharmaceutical Co. Limited, Pfizer Japan, Inc. M.N. received lecture fees from AbbVie GK, Eisai Co., Ltd., EA Pharma Co., Ltd. and Mochida Pharmaceutical Co., Ltd. H.T. received lecture fees from JIMRO Co., Ltd., AbbVie GK, EA Pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Janssen Pharmaceutical K.K., Nikkiso Co., Ltd., Nippon Kayaku Co., Ltd. and Takeda Pharmaceutical; and has received research grants from AbbVie GK, Janssen Pharmaceutical K.K., EA Pharma, Takeda Pharmaceutical, AstraZeneca K.K, and Kissei Pharmaceutical Co., Ltd. T.K. is a member of endowed chair by Miyarisan Pharmaceutical CO., Ltd., JIMRO Co., Ltd., Mochida Pharmaceutical Co., Ltd., and Kyorin Pharmaceutical CO., Ltd. H.N. reports receiving personal fees from Abbvie Inc., Takeda Pharmaceutical CO.,Ltd., Mitsubishi Tanabe Pharm Corperation, Janssen Pharmaceutical K.K, Gilead Sciences Inc., Pfizer Inc., EA Pharma CO.,Ltd., Kyorin Pharmaceutical CO.,Ltd., Mochida Pharmaceutical CO.,Ltd., VIATRIS Inc, JIMRO Co., Ltd., and Daiichi Sankyo Co., Ltd, scholarship grants from Nippon Kayaku Co.,Ltd., Mochida Pharmaceutical CO.,Ltd., Kyorin Pharmaceutical CO.,Ltd., Zeria Pharmaceutical CO.,Ltd., Mitsubishi Tanabe Pharma Corperation, and EA Pharma CO.,Ltd., research grants from Hoya Group Pentax Medical, Abbvie Inc., and endowed chair from Miyarisan Pharmaceutical CO.,LTD.,JIMRO Co.,Ltd., Mochida Pharmaceutical Co.,Ltd., and Kyorin Pharmaceutical CO.,Ltd.
Figures




References
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al.Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2005;353(23):2462-2476. - PubMed
LinkOut - more resources
Full Text Sources
