This is a preprint.
A dynamic compositional equilibrium governs mRNA recognition by eIF3
- PMID: 38712078
- PMCID: PMC11071631
- DOI: 10.1101/2024.04.25.581977
A dynamic compositional equilibrium governs mRNA recognition by eIF3
Abstract
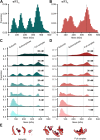
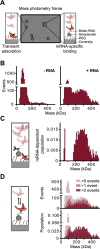
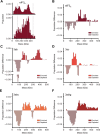
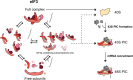
Eukaryotic translation initiation factor (eIF) 3 is a multi-subunit protein complex that binds both ribosomes and messenger RNAs (mRNAs) to drive a diverse set of mechanistic steps during translation of an mRNA into the protein it encodes. And yet, a unifying framework explaining how eIF3 performs these numerous activities is lacking. Using single-molecule light scattering microscopy, we demonstrate that Saccharomyces cerevisiae eIF3 is in dynamic exchange between the full complex, subcomplexes, and subunits. By extending our microscopy approach to an in vitro reconstituted eIF3 and complementing it with biochemical assays, we define the subspecies comprising this dynamic compositional equilibrium and show that mRNA binding by eIF3 is not driven by the full complex but instead by the eIF3a subunit within eIF3a-containing subcomplexes. Our findings provide a mechanistic model for the role of eIF3 in mRNA recruitment and establish a mechanistic framework for explaining and investigating the other activities of eIF3.
Keywords: Translation; biochemistry; biophysics; eIF3; mRNA; mRNA recruitment; mass photometry; ribosome; single-molecule; translation initiation; translational regulation.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
