This is a preprint.
TLR2 Supports γδ T cell IL-17A Response to ocular surface commensals by Metabolic Reprogramming
- PMID: 38712203
- PMCID: PMC11071315
- DOI: 10.1101/2024.04.01.587519
TLR2 Supports γδ T cell IL-17A Response to ocular surface commensals by Metabolic Reprogramming
Update in
-
TLR2 supports γδ T cell IL-17A response to ocular surface commensals by metabolic reprogramming.J Exp Med. 2025 Nov 3;222(11):e20251046. doi: 10.1084/jem.20251046. Epub 2025 Sep 11. J Exp Med. 2025. PMID: 40932624
Abstract
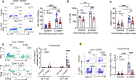
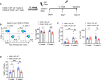
The ocular surface is a mucosal barrier tissue colonized by commensal microbes, which tune local immunity by eliciting IL-17 from conjunctival γδ T cells to prevent pathogenic infection. The commensal Corynebacterium mastitidis (C. mast) elicits protective IL-17 responses from conjunctival Vγ4 T cells through a combination of γδ TCR ligation and IL-1 signaling. Here, we identify Vγ6 T cells as a major C. mast-responsive subset in the conjunctiva and uncover its unique activation requirements. We demonstrate that Vγ6 cells require not only extrinsic (via dendritic cells) but also intrinsic TLR2 stimulation for optimal IL-17A response. Mechanistically, intrinsic TLR2 signaling was associated with epigenetic changes and enhanced expression of genes responsible for metabolic shift to fatty acid oxidation to support Il17a transcription. We identify one key transcription factor, IκBζ, which is upregulated by TLR2 stimulation and is essential for this program. Our study highlights the importance of intrinsic TLR2 signaling in driving metabolic reprogramming and production of IL-17A in microbiome-specific mucosal γδ T cells.
Keywords: FAO; IL-17A; ocular commensal; ΤLR2; γδ Τ cells.
Figures





References
-
- Gomez-Rodriguez J., Wohlfert E.A., Handon R., Meylan F., Wu J.Z., Anderson S.M., Kirby M.R., Belkaid Y., and Schwartzberg P.L. (2014). Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. Journal of Experimental Medicine 211, 529–543. 10.1084/jem.20131459. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
