This is a preprint.
Theta oscillons in behaving rats
- PMID: 38712230
- PMCID: PMC11071438
- DOI: 10.1101/2024.04.21.590487
Theta oscillons in behaving rats
Update in
-
Theta Oscillons in Behaving Rats.J Neurosci. 2025 May 14;45(20):e0164242025. doi: 10.1523/JNEUROSCI.0164-24.2025. J Neurosci. 2025. PMID: 40169263 Free PMC article.
Abstract
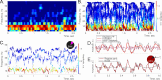
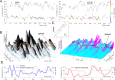
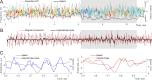
Recently discovered constituents of the brain waves-the oscillons-provide high-resolution representation of the extracellular field dynamics. Here we study the most robust, highest-amplitude oscillons that manifest in actively behaving rats and generally correspond to the traditional -waves. We show that the resemblances between -oscillons and the conventional -waves apply to the ballpark characteristics-mean frequencies, amplitudes, and bandwidths. In addition, both hippocampal and cortical oscillons exhibit a number of intricate, behavior-attuned, transient properties that suggest a new vantage point for understanding the -rhythms' structure, origins and functions. We demonstrate that oscillons are frequency-modulated waves, with speed-controlled parameters, embedded into a noise background. We also use a basic model of neuronal synchronization to contextualize and to interpret the observed phenomena. In particular, we argue that the synchronicity level in physiological networks is fairly weak and modulated by the animal's locomotion.
Figures



References
-
- Buzsáki G. Rhythms in the brain. Oxford University Press, USA, (2011).
-
- Thut G. Miniussi C. & Gross J. The Functional Importance of Rhythmic Activity in the Brain. Current Biology 22: R658 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
