This is a preprint.
Exploring the PET in vivo generator 134Ce as a theranostic match for 225Ac
- PMID: 38712285
- PMCID: PMC11071455
- DOI: 10.1101/2024.04.25.591165
Exploring the PET in vivo generator 134Ce as a theranostic match for 225Ac
Update in
-
Examination of the PET in vivo generator 134Ce as a theranostic match for 225Ac.Eur J Nucl Med Mol Imaging. 2024 Nov;51(13):4015-4025. doi: 10.1007/s00259-024-06811-w. Epub 2024 Jun 28. Eur J Nucl Med Mol Imaging. 2024. PMID: 38940841 Free PMC article.
Abstract
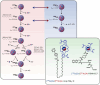
Purpose: The radionuclide pair cerium-134/lanthanum-134 (134Ce/134La) was recently proposed as a suitable diagnostic counterpart for the therapeutic alpha-emitter actinium-225 (225Ac). The unique properties of 134Ce offer perspectives for developing innovative in vivo investigations not possible with 225Ac. In this work, 225Ac- and 134Ce-labeled tracers were directly compared using internalizing and slow-internalizing cancer models to evaluate their in vivo comparability, progeny meandering, and potential as a matched theranostic pair for clinical translation. Despite being an excellent chemical match, 134Ce/134La has limitations to the setting of quantitative positron emission tomography imaging.
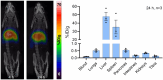
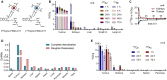
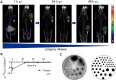
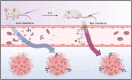
Methods: The precursor PSMA-617 and a macropa-based tetrazine-conjugate (mcp-PEG8-Tz) were radiolabelled with 225Ac or 134Ce and compared in vitro and in vivo using standard (radio)chemical methods. Employing biodistribution studies and positron emission tomography (PET) imaging in athymic nude mice, the radiolabelled PSMA-617 tracers were evaluated in a PC3/PIP (PC3 engineered to express a high level of prostate-specific membrane antigen) prostate cancer mouse model. The 225Ac and 134Ce-labeled mcp-PEG8-Tz were investigated in a BxPC-3 pancreatic tumour model harnessing the pretargeting strategy based on a trans-cyclooctene-modified 5B1 monoclonal antibody.
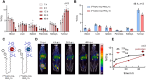
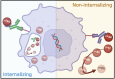
Results: In vitro and in vivo studies with both 225Ac and 134Ce-labelled tracers led to comparable results, confirming the matching pharmacokinetics of this theranostic pair. However, PET imaging of the 134Ce-labelled precursors indicated that quantification is highly dependent on tracer internalization due to the redistribution of 134Ce's PET-compatible daughter 134La. Consequently, radiotracers based on internalizing vectors like PSMA-617 are suited for this theranostic pair, while slow-internalizing 225Ac-labelled tracers are not quantitatively represented by 134Ce PET imaging.
Conclusion: When employing slow-internalizing vectors, 134Ce might not be an ideal match for 225Ac due to the underestimation of tumour uptake caused by the in vivo redistribution of 134La. However, this same characteristic makes it possible to estimate the redistribution of 225Ac's progeny noninvasively. In future studies, this unique PET in vivo generator will further be harnessed to study tracer internalization, trafficking of receptors, and the progression of the tumour microenvironment.
Keywords: Actinium-225; Cerium-134; PET Imaging; Pretargeting; Progeny Release; Targeted Alpha Therapy.
Figures
References
-
- National Isotope Development Center (November 17, 2022). Introducing a new isotope supply chain to the global market: Cerium-134. Accessed April 17, 2024. https://www.isotopes.gov/now_available_Cerium134.
-
- Longtine M, Shim K, Abou D, Summer L, Voller T, Thorek D, Wahl R. Initial evaluation of Cerium-134 (134Ce) for immunoPET. Journal of Nuclear Medicine. 2022;63:2578.
-
- Greenwood RC, Gehrke RJ, Helmer RG, Reich CW, Baker JD. Gamma-ray emission from 134Ce and levels in 134La. Nuclear Physics A. 1976;270:29–44. doi:10.1016/0375-9474(76)90125-1. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
