The gut microbiome in end-stage lung disease and lung transplantation
- PMID: 38712927
- PMCID: PMC11237811
- DOI: 10.1128/msystems.01312-23
The gut microbiome in end-stage lung disease and lung transplantation
Abstract
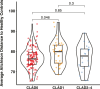
Gut dysbiosis has been associated with impaired outcomes in liver and kidney transplant recipients, but the gut microbiome of lung transplant recipients has not been extensively explored. We assessed the gut microbiome in 64 fecal samples from end-stage lung disease patients before transplantation and 219 samples from lung transplant recipients after transplantation using metagenomic sequencing. To identify dysbiotic microbial signatures, we analyzed 243 fecal samples from age-, sex-, and BMI-matched healthy controls. By unsupervised clustering, we identified five groups of lung transplant recipients using different combinations of immunosuppressants and antibiotics and analyzed them in relation to the gut microbiome. Finally, we investigated the gut microbiome of lung transplant recipients in different chronic lung allograft dysfunction (CLAD) stages and longitudinal gut microbiome changes after transplantation. We found 108 species (58.1%) in end-stage lung disease patients and 139 species (74.7%) in lung transplant recipients that were differentially abundant compared with healthy controls, with several species exhibiting sharp longitudinal increases from before to after transplantation. Different combinations of immunosuppressants and antibiotics were associated with specific gut microbial signatures. We found that the gut microbiome of lung transplant recipients in CLAD stage 0 was more similar to healthy controls compared to those in CLAD stage 1. Finally, the gut microbial diversity of lung transplant recipients remained lower than the average gut microbial diversity of healthy controls up to more than 20 years post-transplantation. Gut dysbiosis, already present before lung transplantation was exacerbated following lung transplantation.IMPORTANCEThis study provides extensive insights into the gut microbiome of end-stage lung disease patients and lung transplant recipients, which warrants further investigation before the gut microbiome can be used for microbiome-targeted interventions that could improve the outcome of lung transplantation.
Keywords: dysbiosis; end-stage lung disease; gut microbiome; immunosuppressive medication; lung transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Hsich E, Meiser B, Potena L, Robinson A, Rossano JW, Sadavarte A, Singh TP, Zuckermann A, Stehlik J, International Society for Heart and Lung Transplantation . 2019. The International thoracic organ transplant registry of the International society for heart and lung transplantation: thirty-sixth adult heart transplantation report — 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 38:1056–1066. doi: 10.1016/j.healun.2019.08.004 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
