Lignin degradation by a novel thermophilic and alkaline yellow laccase from Chitinophaga sp
- PMID: 38712938
- PMCID: PMC11237711
- DOI: 10.1128/spectrum.04013-23
Lignin degradation by a novel thermophilic and alkaline yellow laccase from Chitinophaga sp
Abstract
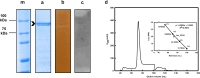
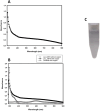
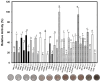
Laccases (EC 1.10.3.2) are oxidoreductases that belong to the multicopper oxidase subfamily and are classified as yellow/white or blue according to their absorption spectrum. Yellow laccases are more useful for industrial processes since they oxidize nonphenolic compounds in the absence of a redox mediator and stand out for being more stable and functional under extreme conditions. This study aimed to characterize a new laccase that was predicted to be present in the genome of Chitinophaga sp. CB10 - Lac_CB10. Lac_CB10, with a molecular mass of 100.06 kDa, was purified and characterized via biochemical assays using guaiacol as a substrate. The enzyme demonstrated extremophilic characteristics, exhibiting relative activity under alkaline conditions (CAPS buffer pH 10.5) and thermophilic conditions (80-90°C), as well as maintaining its activity above 50% for 5 h at 80°C and 90°C. Furthermore, Lac_CB10 presented a spectral profile typical of yellow laccases, exhibiting only one absorbance peak at 300 nm (at the T2/T3 site) and no peak at 600 nm (at the T1 site). When lignin was degraded using copper as an inducer, 52.27% of the material was degraded within 32 h. These results highlight the potential of this enzyme, which is a novel yellow laccase with thermophilic and alkaline activity and the ability to act on lignin. This enzyme could be a valuable addition to the biorefinery process. In addition, this approach has high potential for industrial application and in the bioremediation of contaminated environments since these processes often occur at extreme temperatures and pH values.
Importance: The characterization of the novel yellow laccase, Lac_CB10, derived from Chitinophaga sp. CB10, represents a significant advancement with broad implications. This enzyme displays exceptional stability and functionality under extreme conditions, operating effectively under both alkaline (pH 10.5) and thermophilic (80-90°C) environments. Its capability to maintain considerable activity over extended periods, even at high temperatures, showcases its potential for various industrial applications. Moreover, its distinctive ability to efficiently degrade lignin-demonstrated by a significant 52.27% degradation within 32 h-signifies a promising avenue for biorefinery processes. This newfound laccase's characteristics position it as a crucial asset in the realm of bioremediation, particularly in scenarios involving contamination at extreme pH and temperature levels. The study's findings highlight the enzyme's capacity to address challenges in industrial processes and environmental cleanup, signifying its vital role in advancing biotechnological solutions.
Keywords: extremophile; lignin; yellow laccase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

