Efficient overexpression and purification of severe acute respiratory syndrome coronavirus 2 nucleocapsid proteins in Escherichia coli
- PMID: 38713013
- PMCID: PMC11346444
- DOI: 10.1042/BCJ20240019
Efficient overexpression and purification of severe acute respiratory syndrome coronavirus 2 nucleocapsid proteins in Escherichia coli
Abstract
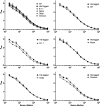
The fundamental biology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid protein (Ncap), its use in diagnostic assays and its potential application as a vaccine component have received considerable attention since the outbreak of the Covid19 pandemic in late 2019. Here we report the scalable expression and purification of soluble, immunologically active, SARS-CoV-2 Ncap in Escherichia coli. Codon-optimised synthetic genes encoding the original Ncap sequence and four common variants with an N-terminal 6His affinity tag (sequence MHHHHHHG) were cloned into an inducible expression vector carrying a regulated bacteriophage T5 synthetic promoter controlled by lac operator binding sites. The constructs were used to express Ncap proteins and protocols developed which allow efficient production of purified Ncap with yields of over 200 mg per litre of culture media. These proteins were deployed in ELISA assays to allow comparison of their responses to human sera. Our results suggest that there was no detectable difference between the 6His-tagged and untagged original Ncap proteins but there may be a slight loss of sensitivity of sera to other Ncap isolates.
Keywords: ELISA; SARS-CoV-2; expression; nucleocapsid; recombinant protein; ribonucleoproteins.
© 2024 The Author(s).
Conflict of interest statement
The University of Sheffield offers some of the proteins described in this manuscript on its commercial licensing portal (https://licensing.sheffield.ac.uk/). The authors declare no other conflicts of interest.
Figures
Similar articles
-
Affinity Tag-Free Purification of SARS-CoV-2 N Protein and Its Crystal Structure in Complex with ssDNA.Biomolecules. 2024 Nov 30;14(12):1538. doi: 10.3390/biom14121538. Biomolecules. 2024. PMID: 39766245 Free PMC article.
-
Expression, purification and immunological characterization of recombinant nucleocapsid protein fragment from SARS-CoV-2.Virology. 2021 May;557:15-22. doi: 10.1016/j.virol.2021.01.004. Epub 2021 Feb 9. Virology. 2021. PMID: 33582454 Free PMC article.
-
Escherichia coli recombinant expression of SARS-CoV-2 protein fragments.Microb Cell Fact. 2022 Feb 5;21(1):21. doi: 10.1186/s12934-022-01753-0. Microb Cell Fact. 2022. PMID: 35123472 Free PMC article.
-
SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights.J Basic Microbiol. 2021 Mar;61(3):180-202. doi: 10.1002/jobm.202000537. Epub 2021 Jan 18. J Basic Microbiol. 2021. PMID: 33460172 Free PMC article. Review.
-
Molecular characterization of SARS-CoV-2 nucleocapsid protein.Front Cell Infect Microbiol. 2024 May 23;14:1415885. doi: 10.3389/fcimb.2024.1415885. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38846351 Free PMC article. Review.
Cited by
-
High SARS-CoV-2 incidence and asymptomatic fraction during Delta and Omicron BA.1 waves in The Gambia.Nat Commun. 2024 May 7;15(1):3814. doi: 10.1038/s41467-024-48098-3. Nat Commun. 2024. PMID: 38714680 Free PMC article.
-
Compartmentalised mucosal and blood immunity to SARS-CoV-2 is associated with high seroprevalence before the Delta wave in Africa.Commun Med (Lond). 2025 May 16;5(1):178. doi: 10.1038/s43856-025-00902-x. Commun Med (Lond). 2025. PMID: 40379979 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

