Evaluation of a Cascaded Deep Learning-based Algorithm for Prostate Lesion Detection at Biparametric MRI
- PMID: 38713024
- PMCID: PMC11140533
- DOI: 10.1148/radiol.230750
Evaluation of a Cascaded Deep Learning-based Algorithm for Prostate Lesion Detection at Biparametric MRI
Abstract
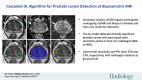
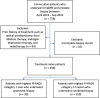
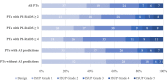
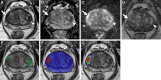
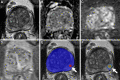
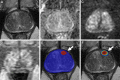
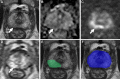
Background Multiparametric MRI (mpMRI) improves prostate cancer (PCa) detection compared with systematic biopsy, but its interpretation is prone to interreader variation, which results in performance inconsistency. Artificial intelligence (AI) models can assist in mpMRI interpretation, but large training data sets and extensive model testing are required. Purpose To evaluate a biparametric MRI AI algorithm for intraprostatic lesion detection and segmentation and to compare its performance with radiologist readings and biopsy results. Materials and Methods This secondary analysis of a prospective registry included consecutive patients with suspected or known PCa who underwent mpMRI, US-guided systematic biopsy, or combined systematic and MRI/US fusion-guided biopsy between April 2019 and September 2022. All lesions were prospectively evaluated using Prostate Imaging Reporting and Data System version 2.1. The lesion- and participant-level performance of a previously developed cascaded deep learning algorithm was compared with histopathologic outcomes and radiologist readings using sensitivity, positive predictive value (PPV), and Dice similarity coefficient (DSC). Results A total of 658 male participants (median age, 67 years [IQR, 61-71 years]) with 1029 MRI-visible lesions were included. At histopathologic analysis, 45% (294 of 658) of participants had lesions of International Society of Urological Pathology (ISUP) grade group (GG) 2 or higher. The algorithm identified 96% (282 of 294; 95% CI: 94%, 98%) of all participants with clinically significant PCa, whereas the radiologist identified 98% (287 of 294; 95% CI: 96%, 99%; P = .23). The algorithm identified 84% (103 of 122), 96% (152 of 159), 96% (47 of 49), 95% (38 of 40), and 98% (45 of 46) of participants with ISUP GG 1, 2, 3, 4, and 5 lesions, respectively. In the lesion-level analysis using radiologist ground truth, the detection sensitivity was 55% (569 of 1029; 95% CI: 52%, 58%), and the PPV was 57% (535 of 934; 95% CI: 54%, 61%). The mean number of false-positive lesions per participant was 0.61 (range, 0-3). The lesion segmentation DSC was 0.29. Conclusion The AI algorithm detected cancer-suspicious lesions on biparametric MRI scans with a performance comparable to that of an experienced radiologist. Moreover, the algorithm reliably predicted clinically significant lesions at histopathologic examination. ClinicalTrials.gov Identifier: NCT03354416 © RSNA, 2024 Supplemental material is available for this article.
Conflict of interest statement
Figures
Similar articles
-
A Cascaded Deep Learning-Based Artificial Intelligence Algorithm for Automated Lesion Detection and Classification on Biparametric Prostate Magnetic Resonance Imaging.Acad Radiol. 2022 Aug;29(8):1159-1168. doi: 10.1016/j.acra.2021.08.019. Epub 2021 Sep 28. Acad Radiol. 2022. PMID: 34598869 Free PMC article.
-
External Validation of a Previously Developed Deep Learning-based Prostate Lesion Detection Algorithm on Paired External and In-House Biparametric MRI Scans.Radiol Imaging Cancer. 2024 Nov;6(6):e240050. doi: 10.1148/rycan.240050. Radiol Imaging Cancer. 2024. PMID: 39400232 Free PMC article.
-
MRI-based Deep Learning Algorithm for Assisting Clinically Significant Prostate Cancer Detection: A Bicenter Prospective Study.Radiology. 2025 Mar;314(3):e232788. doi: 10.1148/radiol.232788. Radiology. 2025. PMID: 40067105
-
Artificial Intelligence in Magnetic Resonance Imaging-based Prostate Cancer Diagnosis: Where Do We Stand in 2021?Eur Urol Focus. 2022 Mar;8(2):409-417. doi: 10.1016/j.euf.2021.03.020. Epub 2021 Mar 25. Eur Urol Focus. 2022. PMID: 33773964 Review.
-
Multiparametric MRI in detection and staging of prostate cancer.Dan Med J. 2017 Feb;64(2):B5327. Dan Med J. 2017. PMID: 28157066 Review.
Cited by
-
Target Volume Optimization for Localized Prostate Cancer.Pract Radiat Oncol. 2024 Nov-Dec;14(6):522-540. doi: 10.1016/j.prro.2024.06.006. Epub 2024 Jul 15. Pract Radiat Oncol. 2024. PMID: 39019208 Free PMC article. Review.
-
Exploring the role of multimodal [18F]F-PSMA-1007 PET/CT and multiparametric MRI data in predicting ISUP grading of primary prostate cancer.Eur J Nucl Med Mol Imaging. 2025 May;52(6):2087-2095. doi: 10.1007/s00259-025-07099-0. Epub 2025 Jan 28. Eur J Nucl Med Mol Imaging. 2025. PMID: 39871017
-
Multimodal approach to optimize biopsy decision-making for PI-RADS 3 lesions on multiparametric MRI.Clin Imaging. 2025 Jan;117:110363. doi: 10.1016/j.clinimag.2024.110363. Epub 2024 Nov 19. Clin Imaging. 2025. PMID: 39579754
-
Prostate Cancer Risk Stratification and Scan Tailoring Using Deep Learning on Abbreviated Prostate MRI.J Magn Reson Imaging. 2025 Sep;62(3):858-866. doi: 10.1002/jmri.29798. Epub 2025 Apr 22. J Magn Reson Imaging. 2025. PMID: 40259798
-
External validation of AI for detecting clinically significant prostate cancer using biparametric MRI.Abdom Radiol (NY). 2025 Feb;50(2):784-793. doi: 10.1007/s00261-024-04560-w. Epub 2024 Sep 3. Abdom Radiol (NY). 2025. PMID: 39225718 No abstract available.
References
-
- Ahmed HU , El-Shater Bosaily A , Brown LC , et al. . Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study . Lancet 2017. ; 389 ( 10071 ): 815 – 822 . - PubMed
-
- Turkbey B , Rosenkrantz AB , Haider MA , et al. . Prostate Imaging Reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2 . Eur Urol 2019. ; 76 ( 3 ): 340 – 351 . - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

