Mitochondrial Calcium Regulation of Cardiac Metabolism in Health and Disease
- PMID: 38713090
- PMCID: PMC11460536
- DOI: 10.1152/physiol.00014.2024
Mitochondrial Calcium Regulation of Cardiac Metabolism in Health and Disease
Abstract
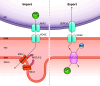
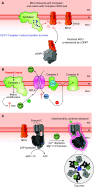
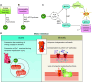
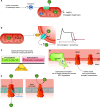
Oxidative phosphorylation is regulated by mitochondrial calcium (Ca2+) in health and disease. In physiological states, Ca2+ enters via the mitochondrial Ca2+ uniporter and rapidly enhances NADH and ATP production. However, maintaining Ca2+ homeostasis is critical: insufficient Ca2+ impairs stress adaptation, and Ca2+ overload can trigger cell death. In this review, we delve into recent insights further defining the relationship between mitochondrial Ca2+ dynamics and oxidative phosphorylation. Our focus is on how such regulation affects cardiac function in health and disease, including heart failure, ischemia-reperfusion, arrhythmias, catecholaminergic polymorphic ventricular tachycardia, mitochondrial cardiomyopathies, Barth syndrome, and Friedreich's ataxia. Several themes emerge from recent data. First, mitochondrial Ca2+ regulation is critical for fuel substrate selection, metabolite import, and matching of ATP supply to demand. Second, mitochondrial Ca2+ regulates both the production and response to reactive oxygen species (ROS), and the balance between its pro- and antioxidant effects is key to how it contributes to physiological and pathological states. Third, Ca2+ exerts localized effects on the electron transport chain (ETC), not through traditional allosteric mechanisms but rather indirectly. These effects hinge on specific transporters, such as the uniporter or the Na+/Ca2+ exchanger, and may not be noticeable acutely, contributing differently to phenotypes depending on whether Ca2+ transporters are acutely or chronically modified. Perturbations in these novel relationships during disease states may either serve as compensatory mechanisms or exacerbate impairments in oxidative phosphorylation. Consequently, targeting mitochondrial Ca2+ holds promise as a therapeutic strategy for a variety of cardiac diseases characterized by contractile failure or arrhythmias.
Keywords: MCU; heart failure; ischemia-reperfusion injury; mitochondrial calcium transport; mitochondrial cardiomyopathy.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Beyond the TCA cycle: new insights into mitochondrial calcium regulation of oxidative phosphorylation.Biochem Soc Trans. 2023 Aug 31;51(4):1661-1673. doi: 10.1042/BST20230012. Biochem Soc Trans. 2023. PMID: 37641565 Free PMC article. Review.
-
Loss of Mitochondrial Ca2+ Uniporter Limits Inotropic Reserve and Provides Trigger and Substrate for Arrhythmias in Barth Syndrome Cardiomyopathy.Circulation. 2021 Nov 23;144(21):1694-1713. doi: 10.1161/CIRCULATIONAHA.121.053755. Epub 2021 Oct 14. Circulation. 2021. PMID: 34648376
-
MCU overexpression evokes disparate dose-dependent effects on mito-ROS and spontaneous Ca2+ release in hypertrophic rat cardiomyocytes.Am J Physiol Heart Circ Physiol. 2021 Oct 1;321(4):H615-H632. doi: 10.1152/ajpheart.00126.2021. Epub 2021 Aug 20. Am J Physiol Heart Circ Physiol. 2021. PMID: 34415186 Free PMC article.
-
Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart.Proc Natl Acad Sci U S A. 2015 Jul 21;112(29):9129-34. doi: 10.1073/pnas.1504705112. Epub 2015 Jul 7. Proc Natl Acad Sci U S A. 2015. PMID: 26153425 Free PMC article.
-
Mitochondrial calcium homeostasis and atrial fibrillation: Mechanisms and therapeutic strategies review.Curr Probl Cardiol. 2025 Mar;50(3):102988. doi: 10.1016/j.cpcardiol.2025.102988. Epub 2025 Jan 17. Curr Probl Cardiol. 2025. PMID: 39828107 Review.
Cited by
-
Protective Effects and Mechanisms of Inhibiting Endoplasmic Reticulum Stress on Cold Seawater Immersion Combined with Hemorrhagic Shock.J Inflamm Res. 2024 Jul 23;17:4923-4940. doi: 10.2147/JIR.S469622. eCollection 2024. J Inflamm Res. 2024. PMID: 39070132 Free PMC article.
-
Causal gene identification using mitochondria-associated genome-wide mendelian randomization in atrial fibrillation.Front Pharmacol. 2024 Jul 29;15:1439816. doi: 10.3389/fphar.2024.1439816. eCollection 2024. Front Pharmacol. 2024. PMID: 39135799 Free PMC article.
-
Association between admission serum calcium levels and in‑hospital mortality in acute myocardial infarction patients with cardiovascular-kidney-metabolic syndrome.Endocrine. 2025 Jul;89(1):232-239. doi: 10.1007/s12020-025-04217-8. Epub 2025 Mar 29. Endocrine. 2025. PMID: 40156686 Free PMC article.
-
Aminooxyacetic acid ameliorates alcohol-induced learning and memory deficits through BDNF-TrkB pathway and calcium homeostasis.Eur J Med Res. 2025 May 5;30(1):365. doi: 10.1186/s40001-025-02630-3. Eur J Med Res. 2025. PMID: 40325392 Free PMC article.
-
Barth Syndrome: TAFAZZIN Gene, Cardiologic Aspects, and Mitochondrial Studies-A Comprehensive Narrative Review.Genes (Basel). 2025 Apr 18;16(4):465. doi: 10.3390/genes16040465. Genes (Basel). 2025. PMID: 40282425 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

