Effect of Therapeutic Drug Monitoring on Adherence and Blood Pressure: A Multicenter Randomized Clinical Trial
- PMID: 38713475
- PMCID: PMC11403020
- DOI: 10.1093/ajh/hpae059
Effect of Therapeutic Drug Monitoring on Adherence and Blood Pressure: A Multicenter Randomized Clinical Trial
Abstract
Background: Drug concentration in blood or urine is an acknowledged method to detect nonadherence. Observational studies suggest that informing patients about low or absent serum drug levels improves blood pressure (BP). We performed a multicenter randomized clinical trial to test the hypothesis that therapeutic drug monitoring (TDM) could improve drug adherence and BP in patients with uncontrolled hypertension (HT).
Methods: Patients were ≥18 years on stable treatment with at least 2 antihypertensive agents. We planned to randomize 80 nonadherent patients with a systolic daytime ambulatory BP ≥135 mm Hg to TDM intervention or not. The control group and the study personnel who measured BP remained uninformed about serum drug measurements throughout. All patients and physicians were blinded for BPs. Lifestyle advice and detailed information on the disease process and the importance of BP treatment were given to both groups.
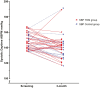
Results: From 2017 to 2022, we randomized 46 diagnosed nonadherent from a total of 606 patients with uncontrolled HT. The TDM group had a 6.7 (±14.5) mm Hg reduction from 147.9 (±10.3) to 141.1 (±14.1) mm Hg, and the control group experienced a 7.3 (±13.2) mm Hg reduction from 147.1 (±9.2) to 139.1 (±17.4) mm Hg, P = 0.9 between groups. Adherence improved in both groups, 73% in the TDM group and 59% in the control group became adherent at 3 months, P = 0.51.
Conclusions: In our prospective multicenter clinical trial of uncontrolled and nonadherent hypertensive patients, we found no additional effect of TDM on BP and drug adherence compared with standard care.
Clinical trials registration: Trial Number NCT03209154, www.clinicaltrials.gov.
Keywords: adherence; antihypertensive drugs; blood pressure; hypertension; nonadherence; randomized clinical trial; therapeutic drug monitoring.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Journal of Hypertension, Ltd.
Conflict of interest statement
S.E.K. reports lecture honoraria from Getz, Emcure, JB Pharma, Merck KGaA, Vector-Intas, and Zydus. A.H. reports lecture honoraria from AstraZeneca. The other authors report no disclosures.
Figures




Comment in
-
Therapeutic Drug Monitoring and the Challenge of Conducting Trials to Improve Antihypertensive Medication Adherence.Am J Hypertens. 2024 Sep 16;37(10):745-747. doi: 10.1093/ajh/hpae075. Am J Hypertens. 2024. PMID: 38832430 Free PMC article. No abstract available.
References
-
- Burnier M, Egan BM.. Adherence in hypertension. Circ Res 2019; 124:1124–1140. - PubMed
-
- Simon ST, Kini V, Levy AE, Ho PM.. Medication adherence in cardiovascular medicine. BMJ 2021; 374:n1493. - PubMed
-
- Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, Azizi M, Benetos A, Borghi C, Hitij JB, Cifkova R, Coca A, Cornelissen V, Cruickshank JK, Cunha PG, Danser AHJ, Pinho RM, Delles C, Dominiczak AF, Dorobantu M, Doumas M, Fernández-Alfonso MS, Halimi J-M, Járai Z, Jelaković B, Jordan J, Kuznetsova T, Laurent S, Lovic D, Lurbe E, Mahfoud F, Manolis A, Miglinas M, Narkiewicz K, Niiranen T, Palatini P, Parati G, Pathak A, Persu A, Polonia J, Redon J, Sarafidis P, Schmieder R, Spronck B, Stabouli S, Stergiou G, Taddei S, Thomopoulos C, Tomaszewski M, Van de Borne P, Wanner C, Weber T, Williams B, Zhang Z-Y, Kjeldsen SE.. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens 2023; 41:1874–2071. - PubMed
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL; Writing Committee Members. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

