SUCNR1 regulates insulin secretion and glucose elevates the succinate response in people with prediabetes
- PMID: 38713514
- PMCID: PMC11178533
- DOI: 10.1172/JCI173214
SUCNR1 regulates insulin secretion and glucose elevates the succinate response in people with prediabetes
Abstract
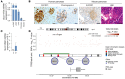
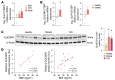
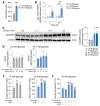
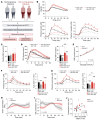
Pancreatic β cell dysfunction is a key feature of type 2 diabetes, and novel regulators of insulin secretion are desirable. Here, we report that succinate receptor 1 (SUCNR1) is expressed in β cells and is upregulated in hyperglycemic states in mice and humans. We found that succinate acted as a hormone-like metabolite and stimulated insulin secretion via a SUCNR1-Gq-PKC-dependent mechanism in human β cells. Mice with β cell-specific Sucnr1 deficiency exhibited impaired glucose tolerance and insulin secretion on a high-fat diet, indicating that SUCNR1 is essential for preserving insulin secretion in diet-induced insulin resistance. Patients with impaired glucose tolerance showed an enhanced nutrition-related succinate response, which correlates with the potentiation of insulin secretion during intravenous glucose administration. These data demonstrate that the succinate/SUCNR1 axis is activated by high glucose and identify a GPCR-mediated amplifying pathway for insulin secretion relevant to the hyperinsulinemia of prediabetic states.
Keywords: Beta cells; Endocrinology; G protein–coupled receptors; Insulin; Metabolism.
Figures