Differential effects of FcRn antagonists on the subcellular trafficking of FcRn and albumin
- PMID: 38713534
- PMCID: PMC11141909
- DOI: 10.1172/jci.insight.176166
Differential effects of FcRn antagonists on the subcellular trafficking of FcRn and albumin
Abstract
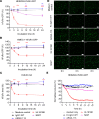
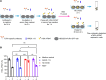
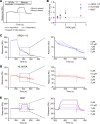
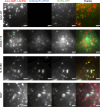
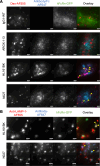
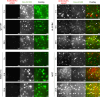
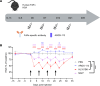
The homeostasis of IgG is maintained by the neonatal Fc receptor, FcRn. Consequently, antagonism of FcRn to reduce endogenous IgG levels is an emerging strategy for treating antibody-mediated autoimmune disorders using either FcRn-specific antibodies or an engineered Fc fragment. For certain FcRn-specific antibodies, this approach has resulted in reductions in the levels of serum albumin, the other major ligand transported by FcRn. Cellular and molecular analyses of a panel of FcRn antagonists have been carried out to elucidate the mechanisms leading to their differential effects on albumin homeostasis. These analyses have identified 2 processes underlying decreases in albumin levels during FcRn blockade: increased degradation of FcRn and competition between antagonist and albumin for FcRn binding. These findings have potential implications for the design of drugs to modulate FcRn function.
Keywords: Autoimmune diseases; Autoimmunity; Immunoglobulins; Immunology.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

