Researching COVID to enhance recovery (RECOVER) pediatric study protocol: Rationale, objectives and design
- PMID: 38713673
- PMCID: PMC11075869
- DOI: 10.1371/journal.pone.0285635
Researching COVID to enhance recovery (RECOVER) pediatric study protocol: Rationale, objectives and design
Abstract
Importance: The prevalence, pathophysiology, and long-term outcomes of COVID-19 (post-acute sequelae of SARS-CoV-2 [PASC] or "Long COVID") in children and young adults remain unknown. Studies must address the urgent need to define PASC, its mechanisms, and potential treatment targets in children and young adults.
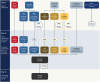
Observations: We describe the protocol for the Pediatric Observational Cohort Study of the NIH's REsearching COVID to Enhance Recovery (RECOVER) Initiative. RECOVER-Pediatrics is an observational meta-cohort study of caregiver-child pairs (birth through 17 years) and young adults (18 through 25 years), recruited from more than 100 sites across the US. This report focuses on two of four cohorts that comprise RECOVER-Pediatrics: 1) a de novo RECOVER prospective cohort of children and young adults with and without previous or current infection; and 2) an extant cohort derived from the Adolescent Brain Cognitive Development (ABCD) study (n = 10,000). The de novo cohort incorporates three tiers of data collection: 1) remote baseline assessments (Tier 1, n = 6000); 2) longitudinal follow-up for up to 4 years (Tier 2, n = 6000); and 3) a subset of participants, primarily the most severely affected by PASC, who will undergo deep phenotyping to explore PASC pathophysiology (Tier 3, n = 600). Youth enrolled in the ABCD study participate in Tier 1. The pediatric protocol was developed as a collaborative partnership of investigators, patients, researchers, clinicians, community partners, and federal partners, intentionally promoting inclusivity and diversity. The protocol is adaptive to facilitate responses to emerging science.
Conclusions and relevance: RECOVER-Pediatrics seeks to characterize the clinical course, underlying mechanisms, and long-term effects of PASC from birth through 25 years old. RECOVER-Pediatrics is designed to elucidate the epidemiology, four-year clinical course, and sociodemographic correlates of pediatric PASC. The data and biosamples will allow examination of mechanistic hypotheses and biomarkers, thus providing insights into potential therapeutic interventions.
Clinical trials.gov identifier: Clinical Trial Registration: http://www.clinicaltrials.gov. Unique identifier: NCT05172011.
Copyright: © 2024 Gross et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Brett Anderson reported receiving direct support for work not related to RECOVER work/publications from Genentech and the National Institute of Allergy and Immunology. Walter Dehority reported receiving grant support from Merck and participating in research for the Moderna COVID-19 pediatric vaccine trial and the Pfizer Paxlovid trial. Alex Fiks reported receiving support from NJM insurance and personal consulting fees not related to this paper from Rutgers University and the American Academy of Pediatrics. Ashraf Harahsheh reported serving as a scientific advisory board member unrelated to this paper for OP2 DRUGS. Lawrence Kleinman reported serving as an unpaid member of the Board of Directors for the DARTNet Institute, as a principle investigator at Quality Matters, Inc., and as the Vice Chair for the Borough of Metuchen Board of Health. Dr. Kleinman also reported grant support for work not related to RECOVER work/publications from NIH, HRSA, and the Robert Wood Johnson Foundation. Dr. Kleinman also reported minority individual stock ownership in Apple Computer, Sanofi SA, Experion, GlaxoSmithKline, Magyar Bank, Regeneron Pharmaceuticals, JP Morgan Chase, and Amgen Inc. Torri Metz reported participating as a Principle Investigator in the medical advisory board for the planning of a Pfizer clinical trial of SARS-CoV-2 vaccination in pregnancy. She is also a principle investigator for a Pfizer study evaluating the pharmacokinetics of Paxlovid in pregnant people with COVID-19. Joshua Milner reported serving as a member of the Scientific Advisory Board for Blueprint Medicines, in a capacity unrelated to RECOVER work/publications. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Centers for Disease Control and Prevention (CDC). Long COVID or Post-COVID Conditions 2023 [cited 2023 September 28, 2023]. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard 2023 2023. https://covid19.who.int/.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous