Association of NOTCH3 Variant Risk Category With 2-Year Clinical and Radiologic Small Vessel Disease Progression in Patients With CADASIL
- PMID: 38713890
- PMCID: PMC11177591
- DOI: 10.1212/WNL.0000000000209310
Association of NOTCH3 Variant Risk Category With 2-Year Clinical and Radiologic Small Vessel Disease Progression in Patients With CADASIL
Abstract
Background and objectives: Pathogenic variants in NOTCH3 are the main cause of hereditary cerebral small vessel disease (SVD). SVD-associated NOTCH3 variants have recently been categorized into high risk (HR), moderate risk (MR), or low risk (LR) for developing early-onset severe SVD. The most severe NOTCH3-associated SVD phenotype is also known as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). We aimed to investigate whether NOTCH3 variant risk category is associated with 2-year progression rate of SVD clinical and neuroimaging outcomes in CADASIL.
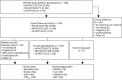
Methods: A single-center prospective 2-year follow-up study was performed of patients with CADASIL. Clinical outcomes were incident stroke, disability (modified Rankin Scale), and executive function (Trail Making Test B given A t-scores). Neuroimaging outcomes were mean skeletonized mean diffusivity (MSMD), normalized white matter hyperintensity volume (nWMHv), normalized lacune volume (nLV), and brain parenchymal fraction (BPF). Cox regression and mixed-effect models, adjusted for age, sex, and cardiovascular risk factors, were used to study 2-year changes in outcomes and differences in disease progression between patients with HR-NOTCH3 and MR-NOTCH3 variants.
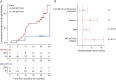
Results: One hundred sixty-two patients with HR (n = 90), MR (n = 67), and LR (n = 5) NOTCH3 variants were included. For the entire cohort, there was 2-year mean progression for MSMD (β = 0.20, 95% CI 0.17-0.23, p = 7.0 × 10-24), nLV (β = 0.13, 95% CI 0.080-0.19, p = 2.1 × 10-6), nWMHv (β = 0.092, 95% CI 0.075-0.11, p = 8.8 × 10-20), and BPF (β = -0.22, 95% CI -0.26 to -0.19, p = 3.2 × 10-22), as well as an increase in disability (p = 0.002) and decline of executive function (β = -0.15, 95% CI -0.30 to -3.4 × 10-5, p = 0.05). The HR-NOTCH3 group had a higher probability of 2-year incident stroke (hazard ratio 4.3, 95% CI 1.4-13.5, p = 0.011), and a higher increase in MSMD (β = 0.074, 95% CI 0.013-0.14, p = 0.017) and nLV (β = 0.14, 95% CI 0.034-0.24, p = 0.0089) than the MR-NOTCH3 group. Subgroup analyses showed significant 2-year progression of MSMD in young (n = 17, β = 0.014, 95% CI 0.0093-0.019, p = 1.4 × 10-5) and premanifest (n = 24, β = 0.012, 95% CI 0.0082-0.016, p = 1.1 × 10-6) individuals.
Discussion: In a trial-sensitive time span of 2 years, we found that patients with HR-NOTCH3 variants have a significantly faster progression of major clinical and neuroimaging outcomes, compared with patients with MR-NOTCH3 variants. This has important implications for clinical trial design and disease prediction and monitoring in the clinic. Moreover, we show that MSMD is a promising outcome measure for trials enrolling premanifest individuals.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures


References
-
- Cho BPH, Nannoni S, Harshfield EL, et al. . NOTCH3 variants are more common than expected in the general population and associated with stroke and vascular dementia: an analysis of 200 000 participants. J Neurol Neurosurg Psychiatry. 2021;92(7):694-701. doi:10.1136/jnnp-2020-325838 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
