Neoantigen landscape supports feasibility of personalized cancer vaccine for follicular lymphoma
- PMID: 38713894
- PMCID: PMC11339042
- DOI: 10.1182/bloodadvances.2022007792
Neoantigen landscape supports feasibility of personalized cancer vaccine for follicular lymphoma
Abstract
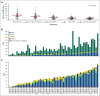
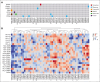
Personalized cancer vaccines designed to target neoantigens represent a promising new treatment paradigm in oncology. In contrast to classical idiotype vaccines, we hypothesized that "polyvalent" vaccines could be engineered for the personalized treatment of follicular lymphoma (FL) using neoantigen discovery by combined whole-exome sequencing (WES) and RNA sequencing (RNA-seq). Fifty-eight tumor samples from 57 patients with FL underwent WES and RNA-seq. Somatic and B-cell clonotype neoantigens were predicted and filtered to identify high-quality neoantigens. B-cell clonality was determined by the alignment of B-cell receptor (BCR) CDR3 regions from RNA-seq data, grouping at the protein level, and comparison with the BCR repertoire from healthy individuals using RNA-seq data. An average of 52 somatic mutations per patient (range, 2-172) were identified, and ≥2 (median, 15) high-quality neoantigens were predicted for 56 of 58 FL samples. The predicted neoantigen peptides were composed of missense mutations (77%), indels (9%), gene fusions (3%), and BCR sequences (11%). Building off of these preclinical analyses, we initiated a pilot clinical trial using personalized neoantigen vaccination combined with PD-1 blockade in patients with relapsed or refractory FL (#NCT03121677). Synthetic long peptide vaccines targeting predicted high-quality neoantigens were successfully synthesized for and administered to all 4 patients enrolled. Initial results demonstrate feasibility, safety, and potential immunologic and clinical responses. Our study suggests that a genomics-driven personalized cancer vaccine strategy is feasible for patients with FL, and this may overcome prior challenges in the field. This trial was registered at www.ClinicalTrials.gov as #NCT03121677.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: N.M.-S. has served as a consultant for Kyowa Hakka Kirin, Daiichi Sankyo, Karyopharm Therapeutics, and C4 Therapeutics; and has institutional research funding from Celgene, Bristol Myers Squibb, Verastem Oncology, Innate Pharmaceuticals, Corvus Pharmaceuticals, and Genentech/Roche. F.F., O.K., V.B., A.B., E.O., and R.A. are full time employees of BostonGene Corporation. D.A.R.-G. has institutional research funding from Genentech; and has served on an advisory board for AstraZeneca. N.L.B. has research funding from ADC Therapeutics, Affimed, Autolus, Bristol Myers Squibb, Celgene, Forty Seven Immune Design, Janssen, Kite Pharma, Merck, Millennium, Pfizer, Pharmacyclics, Roche/Genentech, and Seagen; and has been on the advisory board for ADC Therapeutics, Roche/Genentech, Seagen, BTG, and Acerta. T.A.F. has research funding from ImmunityBio, Affimed, Wugen, and HCW Biologics; consults for Wugen, Gamida Cell, Takeda, Nkarta, Indapta, and Orca Bio; and has equity and potential royalty interest in Wugen. M.G. and O.L.G. have received consulting fees from Rare Cancer Research Foundation and H37 Foundation. M.G., O.L.G., and K.S. have received consulting fees from Jaime Leandro Foundation for their work in neoantigen vaccine design. The remaining authors declare no competing financial interests.
Figures





References
-
- The Non-Hodgkin's Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909–3918. - PubMed
-
- Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055–2063. - PubMed
-
- Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362(9383):516–522. - PubMed
-
- Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99(9):706–714. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

