MRI morphometry of the anterior and posterior cerebellar vermis and its relationship to sensorimotor and cognitive functions in children
- PMID: 38713999
- PMCID: PMC11096723
- DOI: 10.1016/j.dcn.2024.101385
MRI morphometry of the anterior and posterior cerebellar vermis and its relationship to sensorimotor and cognitive functions in children
Abstract
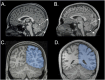
Introduction: The human cerebellum emerges as a posterior brain structure integrating neural networks for sensorimotor, cognitive, and emotional processing across the lifespan. Developmental studies of the cerebellar anatomy and function are scant. We examine age-dependent MRI morphometry of the anterior cerebellar vermis, lobules I-V and posterior neocortical lobules VI-VII and their relationship to sensorimotor and cognitive functions.
Methods: Typically developing children (TDC; n=38; age 9-15) and healthy adults (HAC; n=31; 18-40) participated in high-resolution MRI. Rigorous anatomically informed morphometry of the vermis lobules I-V and VI-VII and total brain volume (TBV) employed manual segmentation computer-assisted FreeSurfer Image Analysis Program [http://surfer.nmr.mgh.harvard.edu]. The neuropsychological scores (WASI-II) were normalized and related to volumes of anterior, posterior vermis, and TBV.
Results: TBVs were age independent. Volumes of I-V and VI-VII were significantly reduced in TDC. The ratio of VI-VII to I-V (∼60%) was stable across age-groups; I-V correlated with visual-spatial-motor skills; VI-VII with verbal, visual-abstract and FSIQ.
Conclusions: In TDC neither anterior I-V nor posterior VI-VII vermis attained adult volumes. The "inverted U" developmental trajectory of gray matter peaking in adolescence does not explain this finding. The hypothesis of protracted development of oligodendrocyte/myelination is suggested as a contributor to TDC's lower cerebellar vermis volumes.
Keywords: Cerebellar vermis; Hypothesis of protracted development of oligodendrocyte/myelination; MRI morphometry; Sensorimotor and cognitive functions; Typically developing children.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. KRC
Figures

References
-
- Aldinger K.A., Thomson Z., Phelps I.G., Haldipur P., Deng M., Timms A.E., Hirano M., Santpere G., Roco C., Rosenberg A.B., Lorente-Galdos B., Gulden F.O., O’Day D., Overman L.M., Lisgo S.N., Alexandre P., Sestan N., Doherty D., Dobyns W.B.…Millen K.J. Spatial and cell type transcriptional landscape of human cerebellar development. Nat. Neurosci. 2021;24(8):8. doi: 10.1038/s41593-021-00872-y. (Article) - DOI - PMC - PubMed
-
- Amore G., Spoto G., Ieni A., Vetri L., Quatrosi G., Di Rosa G., Nicotera A.G. A Focus on the Cerebellum: From Embryogenesis to an Age-Related Clinical Perspective. Front. Syst. Neurosci. 2021;15 〈https://www.frontiersin.org/articles/10.3389/fnsys.2021.646052〉 - DOI - PMC - PubMed
-
- Amso D., Johnson S.P. Selection and inhibition in infancy: Evidence from the spatial negative priming paradigm. Cognition. 2005;95(2):B27–B36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

