CRISPR-dCas13d-based deep screening of proximal and distal splicing-regulatory elements
- PMID: 38714659
- PMCID: PMC11076525
- DOI: 10.1038/s41467-024-47140-8
CRISPR-dCas13d-based deep screening of proximal and distal splicing-regulatory elements
Abstract
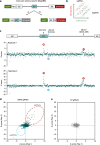
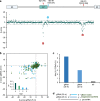
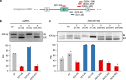
Pre-mRNA splicing, a key process in gene expression, can be therapeutically modulated using various drug modalities, including antisense oligonucleotides (ASOs). However, determining promising targets is hampered by the challenge of systematically mapping splicing-regulatory elements (SREs) in their native sequence context. Here, we use the catalytically inactive CRISPR-RfxCas13d RNA-targeting system (dCas13d/gRNA) as a programmable platform to bind SREs and modulate splicing by competing against endogenous splicing factors. SpliceRUSH, a high-throughput screening method, was developed to map SREs in any gene of interest using a lentivirus gRNA library that tiles the genetic region, including distal intronic sequences. When applied to SMN2, a therapeutic target for spinal muscular atrophy, SpliceRUSH robustly identifies not only known SREs but also a previously unknown distal intronic SRE, which can be targeted to alter exon 7 splicing using either dCas13d/gRNA or ASOs. This technology enables a deeper understanding of splicing regulation with applications for RNA-based drug discovery.
© 2024. The Author(s).
Conflict of interest statement
Y.R., D.U., and C.Z. are inventors on a patent application submitted based on this work. C.Z. is a co-founder of DAYI Therapeutics, Inc. The other authors declare no competing interests.
Figures



Update of
-
Deep screening of proximal and distal splicing-regulatory elements in a native sequence context.bioRxiv [Preprint]. 2023 Aug 21:2023.08.21.554109. doi: 10.1101/2023.08.21.554109. bioRxiv. 2023. Update in: Nat Commun. 2024 May 7;15(1):3839. doi: 10.1038/s41467-024-47140-8. PMID: 37662340 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

