Aging-regulated PNUTS maintains endothelial barrier function via SEMA3B suppression
- PMID: 38714838
- PMCID: PMC11076560
- DOI: 10.1038/s42003-024-06230-5
Aging-regulated PNUTS maintains endothelial barrier function via SEMA3B suppression
Abstract
Age-related diseases pose great challenges to health care systems worldwide. During aging, endothelial senescence increases the risk for cardiovascular disease. Recently, it was described that Phosphatase 1 Nuclear Targeting Subunit (PNUTS) has a central role in cardiomyocyte aging and homeostasis. Here, we determine the role of PNUTS in endothelial cell aging. We confirm that PNUTS is repressed in senescent endothelial cells (ECs). Moreover, PNUTS silencing elicits several of the hallmarks of endothelial aging: senescence, reduced angiogenesis and loss of barrier function. Findings are validate in vivo using endothelial-specific inducible PNUTS-deficient mice (Cdh5-CreERT2;PNUTSfl/fl), termed PNUTSEC-KO. Two weeks after PNUTS deletion, PNUTSEC-KO mice present severe multiorgan failure and vascular leakage. Transcriptomic analysis of PNUTS-silenced HUVECs and lungs of PNUTSEC-KO mice reveal that the PNUTS-PP1 axis tightly regulates the expression of semaphorin 3B (SEMA3B). Indeed, silencing of SEMA3B completely restores barrier function after PNUTS loss-of-function. These results reveal a pivotal role for PNUTS in endothelial homeostasis through a SEMA3B downstream pathway that provides a potential target against the effects of aging in ECs.
© 2024. The Author(s).
Conflict of interest statement
N.L.-V. and L.S. are currently employed by AstraZeneca. All other authors declare no competing interests.
Figures

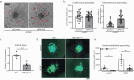
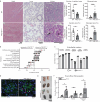
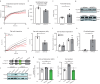
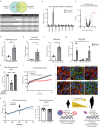
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

