STR mutations on chromosome 15q cause thyrotropin resistance by activating a primate-specific enhancer of MIR7-2/MIR1179
- PMID: 38714869
- PMCID: PMC11472772
- DOI: 10.1038/s41588-024-01717-7
STR mutations on chromosome 15q cause thyrotropin resistance by activating a primate-specific enhancer of MIR7-2/MIR1179
Abstract
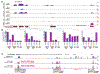
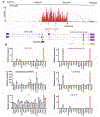
Thyrotropin (TSH) is the master regulator of thyroid gland growth and function. Resistance to TSH (RTSH) describes conditions with reduced sensitivity to TSH. Dominantly inherited RTSH has been linked to a locus on chromosome 15q, but its genetic basis has remained elusive. Here we show that non-coding mutations in a (TTTG)4 short tandem repeat (STR) underlie dominantly inherited RTSH in all 82 affected participants from 12 unrelated families. The STR is contained in a primate-specific Alu retrotransposon with thyroid-specific cis-regulatory chromatin features. Fiber-seq and RNA-seq studies revealed that the mutant STR activates a thyroid-specific enhancer cluster, leading to haplotype-specific upregulation of the bicistronic MIR7-2/MIR1179 locus 35 kb downstream and overexpression of its microRNA products in the participants' thyrocytes. An imbalance in signaling pathways targeted by these micro-RNAs provides a working model for this cause of RTSH. This finding broadens our current knowledge of genetic defects altering pituitary-thyroid feedback regulation.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
A.B.S. is a co-inventor on a patent relating to the Fiber-seq method. The other authors declare no competing interests.
Figures












References
-
- Peters C, van Trotsenburg ASP & Schoenmakers N Diagnosis of endocrine disease: congenital hypothyroidism: update and perspectives. Eur. J. Endocrinol 179, R297–R317 (2018). - PubMed
-
- Boersma B, Otten BJ, Stoelinga GB & Wit JM Catch-up growth after prolonged hypothyroidism. Eur. J. Pediatr 155, 362–367 (1996). - PubMed
-
- Persani L et al. Genetics and phenomics of hypothyroidism due to TSH resistance. Mol. Cell. Endocrinol 322, 72–82 (2010). - PubMed
-
- Stanbury JB, Rocmans P, Buhler UK & Ochi Y Congenital hypothyroidism with impaired thyroid response to thyrotropin. N. Engl. J. Med 279, 1132–1136 (1968). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

