Multiomics analysis identifies oxidative phosphorylation as a cancer vulnerability arising from myristoylation inhibition
- PMID: 38715059
- PMCID: PMC11075276
- DOI: 10.1186/s12967-024-05150-6
Multiomics analysis identifies oxidative phosphorylation as a cancer vulnerability arising from myristoylation inhibition
Abstract
Background: In humans, two ubiquitously expressed N-myristoyltransferases, NMT1 and NMT2, catalyze myristate transfer to proteins to facilitate membrane targeting and signaling. We investigated the expression of NMTs in numerous cancers and found that NMT2 levels are dysregulated by epigenetic suppression, particularly so in hematologic malignancies. This suggests that pharmacological inhibition of the remaining NMT1 could allow for the selective killing of these cells, sparing normal cells with both NMTs.
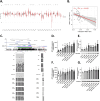
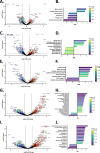
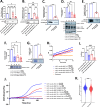
Methods and results: Transcriptomic analysis of 1200 NMT inhibitor (NMTI)-treated cancer cell lines revealed that NMTI sensitivity relates not only to NMT2 loss or NMT1 dependency, but also correlates with a myristoylation inhibition sensitivity signature comprising 54 genes (MISS-54) enriched in hematologic cancers as well as testis, brain, lung, ovary, and colon cancers. Because non-myristoylated proteins are degraded by a glycine-specific N-degron, differential proteomics revealed the major impact of abrogating NMT1 genetically using CRISPR/Cas9 in cancer cells was surprisingly to reduce mitochondrial respiratory complex I proteins rather than cell signaling proteins, some of which were also reduced, albeit to a lesser extent. Cancer cell treatments with the first-in-class NMTI PCLX-001 (zelenirstat), which is undergoing human phase 1/2a trials in advanced lymphoma and solid tumors, recapitulated these effects. The most downregulated myristoylated mitochondrial protein was NDUFAF4, a complex I assembly factor. Knockout of NDUFAF4 or in vitro cell treatment with zelenirstat resulted in loss of complex I, oxidative phosphorylation and respiration, which impacted metabolomes.
Conclusions: Targeting of both, oxidative phosphorylation and cell signaling partly explains the lethal effects of zelenirstat in select cancer types. While the prognostic value of the sensitivity score MISS-54 remains to be validated in patients, our findings continue to warrant the clinical development of zelenirstat as cancer treatment.
Keywords: Cancer; Complex I; N-myristoylation; N-myristoyltransferase; NDUFAF4; NMT inhibitor (NMTI); Oxidative phosphorylation; PCLX-001 (zelenirstat); Respiration.
© 2024. The Author(s).
Conflict of interest statement
As co-founders and/or shareholders of Pacylex Pharmaceuticals Inc. (
Figures




References
-
- Yang SH, Shrivastav A, Kosinski C, Sharma RK, Chen MH, Berthiaume LG, Peters LL, Chuang PT, Young SG, Bergo MO. N-myristoyltransferase 1 is essential in early mouse development. J Biol Chem. 2005;280:18990–18995. - PubMed
-
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. - PubMed
-
- Taniguchi H. Protein myristoylation in protein-lipid and protein-protein interactions. Biophys Chem. 1999;82:129–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

