Neuroinflammation is associated with Alzheimer's disease co-pathology in dementia with Lewy bodies
- PMID: 38715119
- PMCID: PMC11075309
- DOI: 10.1186/s40478-024-01786-z
Neuroinflammation is associated with Alzheimer's disease co-pathology in dementia with Lewy bodies
Abstract
Background: Neuroinflammation and Alzheimer's disease (AD) co-pathology may contribute to disease progression and severity in dementia with Lewy bodies (DLB). This study aims to clarify whether a different pattern of neuroinflammation, such as alteration in microglial and astroglial morphology and distribution, is present in DLB cases with and without AD co-pathology.
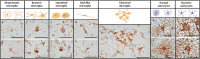
Methods: The morphology and load (% area of immunopositivity) of total (Iba1) and reactive microglia (CD68 and HLA-DR), reactive astrocytes (GFAP) and proteinopathies of alpha-synuclein (KM51/pser129), amyloid-beta (6 F/3D) and p-tau (AT8) were assessed in a cohort of mixed DLB + AD (n = 35), pure DLB (n = 15), pure AD (n = 16) and control (n = 11) donors in limbic and neocortical brain regions using immunostaining, quantitative image analysis and confocal microscopy. Regional and group differences were estimated using a linear mixed model analysis.
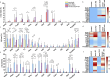
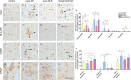
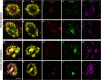
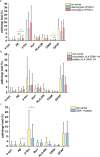
Results: Morphologically, reactive and amoeboid microglia were common in mixed DLB + AD, while homeostatic microglia with a small soma and thin processes were observed in pure DLB cases. A higher density of swollen astrocytes was observed in pure AD cases, but not in mixed DLB + AD or pure DLB cases. Mixed DLB + AD had higher CD68-loads in the amygdala and parahippocampal gyrus than pure DLB cases, but did not differ in astrocytic loads. Pure AD showed higher Iba1-loads in the CA1 and CA2, higher CD68-loads in the CA2 and subiculum, and a higher astrocytic load in the CA1-4 and subiculum than mixed DLB + AD cases. In mixed DLB + AD cases, microglial load associated strongly with amyloid-beta (Iba1, CD68 and HLA-DR), and p-tau (CD68 and HLA-DR), and minimally with alpha-synuclein load (CD68). In addition, the highest microglial activity was found in the amygdala and CA2, and astroglial load in the CA4. Confocal microscopy demonstrated co-localization of large amoeboid microglia with neuritic and classic-cored plaques of amyloid-beta and p-tau in mixed DLB + AD cases.
Conclusions: In conclusion, microglial activation in DLB was largely associated with AD co-pathology, while astrocytic response in DLB was not. In addition, microglial activity was high in limbic regions, with prevalent AD pathology. Our study provides novel insights into the molecular neuropathology of DLB, highlighting the importance of microglial activation in mixed DLB + AD.
Keywords: Alpha-synuclein; Alzheimer’s disease; Amyloid-beta; Astroglia; Co-pathology; Dementia with Lewy bodies; Immunohistochemistry; Lewy body disease; Microglia; Neuroinflammation; Phosphorylated-tau; Post-mortem.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests for this study. WvdB was financially supported by grants from Dutch Research council (ZonMW 70-73305-98-106; 70-73305-98-102; 40-46000-98-101), Alzheimer association (AARF-18-566459), MJ Fox foundation (17253), Parkinson Association (2020-G01), Health Holland and Stichting Woelse Waard. WvdB performed contract research for Roche Tissue Diagnostics, Discoveric Bio and Genentech, and received research consumables from Hoffmann-La Roche and Prothena.
Figures

References
-
- Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, Attems J. Neuropathologically mixed Alzheimer’s and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015;129(5):729–748. doi: 10.1007/s00401-015-1406-3. - DOI - PubMed
-
- Geut H, Vergouw LJM, Galis Y, Ingrassia A, de Jong FJ, Quadri M, van de Berg WDJ. Neuropathological and genetic characteristics of a post-mortem series of cases with dementia with Lewy bodies clinically suspected of Creutzfeldt-Jakob’s disease. Parkinsonism Relat Disord. 2019;63:162–168. doi: 10.1016/j.parkreldis.2019.02.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

