Quantifying cell cycle regulation by tissue crowding
- PMID: 38715360
- PMCID: PMC11947467
- DOI: 10.1016/j.bpj.2024.05.003
Quantifying cell cycle regulation by tissue crowding
Abstract
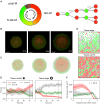
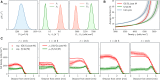
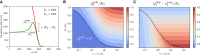
The spatiotemporal coordination and regulation of cell proliferation is fundamental in many aspects of development and tissue maintenance. Cells have the ability to adapt their division rates in response to mechanical constraints, yet we do not fully understand how cell proliferation regulation impacts cell migration phenomena. Here, we present a minimal continuum model of cell migration with cell cycle dynamics, which includes density-dependent effects and hence can account for cell proliferation regulation. By combining minimal mathematical modeling, Bayesian inference, and recent experimental data, we quantify the impact of tissue crowding across different cell cycle stages in epithelial tissue expansion experiments. Our model suggests that cells sense local density and adapt cell cycle progression in response, during G1 and the combined S/G2/M phases, providing an explicit relationship between each cell-cycle-stage duration and local tissue density, which is consistent with several experimental observations. Finally, we compare our mathematical model's predictions to different experiments studying cell cycle regulation and present a quantitative analysis on the impact of density-dependent regulation on cell migration patterns. Our work presents a systematic approach for investigating and analyzing cell cycle data, providing mechanistic insights into how individual cells regulate proliferation, based on population-based experimental measurements.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Jorgensen P., Tyers M. How cells coordinate growth and division. Curr. Biol. 2004;14:R1014–R1027. - PubMed
-
- Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. - PubMed
-
- McClatchey A.I., Yap A.S. Contact inhibition (of proliferation) redux. Curr. Opin. Cell Biol. 2012;24:685–694. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

