Development of an mRNA-based therapeutic vaccine mHTV-03E2 for high-risk HPV-related malignancies
- PMID: 38715363
- PMCID: PMC11286823
- DOI: 10.1016/j.ymthe.2024.04.036
Development of an mRNA-based therapeutic vaccine mHTV-03E2 for high-risk HPV-related malignancies
Abstract
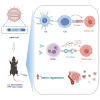
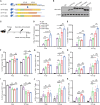
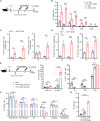
Human papillomavirus (HPV) 16 and 18 infections are related to many human cancers. Despite several preventive vaccines for high-risk (hr) HPVs, there is still an urgent need to develop therapeutic HPV vaccines for targeting pre-existing hrHPV infections and lesions. In this study, we developed a lipid nanoparticle (LNP)-formulated mRNA-based HPV therapeutic vaccine (mHTV)-03E2, simultaneously targeting the E2/E6/E7 of both HPV16 and HPV18. mHTV-03E2 dramatically induced antigen-specific cellular immune responses, leading to significant CD8+ T cell infiltration and cytotoxicity in TC-1 tumors derived from primary lung epithelial cells of C57BL/6 mice expressing HPV E6/E7 antigens, mediated significant tumor regression, and prolonged animal survival, in a dose-dependent manner. We further demonstrated significant T cell immunity against HPV16/18 E6/E7 antigens for up to 4 months post-vaccination in immunological and distant tumor rechallenging experiments, suggesting robust memory T cell immunity against relapse. Finally, mHTV-03E2 synergized with immune checkpoint blockade to inhibit tumor growth and extend animal survival, indicating the potential in combination therapy. We conclude that mHTV-03E2 is an excellent candidate therapeutic mRNA vaccine for treating malignancies caused by HPV16 or HPV18 infections.
Keywords: HPV; animal model; checkpoint blockade; mRNA therapeutic vaccine; tumor.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.C., J.W., W.Z., and Y.D. are co-inventors on pending patent applications related to the HPV-associated mRNA vaccine. H.L., H.Z., H.C., Y.W., T.Z., Y.C., Q.W., J.Z., C.H., Y.D., and W.Z. are employees of RinuaGene Biotechnology Co., Ltd.
Figures





References
-
- Metz C.K., Skof A.S., Sehouli J., Siedentopf J.P., Gebert P., Weiss F., Alba Alejandre I., Heinrich-Rohr M., Weizsaecker K., Henrich W., et al. Assessment of high-risk human papillomavirus infections and associated cervical dysplasia in HIV-positive pregnant women in Germany: a prospective cross-sectional two-centre study. Arch. Gynecol. Obstet. 2023;308:207–218. - PubMed
-
- Alhamlan F.S., Alfageeh M.B., Al Mushait M.A., Al-Badawi I.A., Al-Ahdal M.N. Human Papillomavirus-Associated Cancers. Adv. Exp. Med. Biol. 2021;1313:1–14. - PubMed
-
- Nyitray A.G., Iannacone M.R. The epidemiology of human papillomaviruses. Curr. Probl. Dermatol. 2014;45:75–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

