B cells in the pneumococcus-infected lung are heterogeneous and require CD4+ T cell help including CD40L to become resident memory B cells
- PMID: 38715601
- PMCID: PMC11074383
- DOI: 10.3389/fimmu.2024.1382638
B cells in the pneumococcus-infected lung are heterogeneous and require CD4+ T cell help including CD40L to become resident memory B cells
Abstract
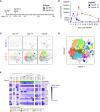
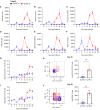
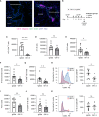
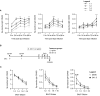
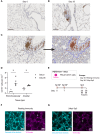
Recovery from respiratory pneumococcal infections generates lung-localized protection against heterotypic bacteria, mediated by resident memory lymphocytes. Optimal protection in mice requires re-exposure to pneumococcus within days of initial infection. Serial surface marker phenotyping of B cell populations in a model of pneumococcal heterotypic immunity revealed that bacterial re-exposure stimulates the immediate accumulation of dynamic and heterogeneous populations of B cells in the lung, and is essential for the establishment of lung resident memory B (BRM) cells. The B cells in the early wave were activated, proliferating locally, and associated with both CD4+ T cells and CXCL13. Antagonist- and antibody-mediated interventions were implemented during this early timeframe to demonstrate that lymphocyte recirculation, CD4+ cells, and CD40 ligand (CD40L) signaling were all needed for lung BRM cell establishment, whereas CXCL13 signaling was not. While most prominent as aggregates in the loose connective tissue of bronchovascular bundles, morphometry and live lung imaging analyses showed that lung BRM cells were equally numerous as single cells dispersed throughout the alveolar septae. We propose that CD40L signaling from antigen-stimulated CD4+ T cells in the infected lung is critical to establishment of local BRM cells, which subsequently protect the airways and parenchyma against future potential infections.
Keywords: B cells; adaptive immunity; lung immunology; mucosal immunity; pneumonia.
Copyright © 2024 Etesami, Barker, Shenoy, De Ana, Arafa, Grifno, Matschulat, Vannini, Pihl, Breen, Soucy, Goltry, Ha, Betsuyaku, Browning, Varelas, Traber, Jones, Quinton, Maglione, Nia, Belkina and Mizgerd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL165718/HL/NHLBI NIH HHS/United States
- R35 HL135756/HL/NHLBI NIH HHS/United States
- R33 HL137081/HL/NHLBI NIH HHS/United States
- R00 HL157555/HL/NHLBI NIH HHS/United States
- R01 HL171499/HL/NHLBI NIH HHS/United States
- F30 HL158109/HL/NHLBI NIH HHS/United States
- R01 AI162850/AI/NIAID NIH HHS/United States
- DP2 HL168562/HL/NHLBI NIH HHS/United States
- R01 AI115053/AI/NIAID NIH HHS/United States
- T32 HL007035/HL/NHLBI NIH HHS/United States
- R01 HL158732/HL/NHLBI NIH HHS/United States
- K99 HL157555/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

