Model discovery approach enables noninvasive measurement of intra-tumoral fluid transport in dynamic MRI
- PMID: 38715647
- PMCID: PMC11075764
- DOI: 10.1063/5.0190561
Model discovery approach enables noninvasive measurement of intra-tumoral fluid transport in dynamic MRI
Abstract
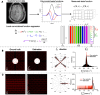
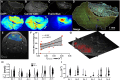
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a routine method to noninvasively quantify perfusion dynamics in tissues. The standard practice for analyzing DCE-MRI data is to fit an ordinary differential equation to each voxel. Recent advances in data science provide an opportunity to move beyond existing methods to obtain more accurate measurements of fluid properties. Here, we developed a localized convolutional function regression that enables simultaneous measurement of interstitial fluid velocity, diffusion, and perfusion in 3D. We validated the method computationally and experimentally, demonstrating accurate measurement of fluid dynamics in situ and in vivo. Applying the method to human MRIs, we observed tissue-specific differences in fluid dynamics, with an increased fluid velocity in breast cancer as compared to brain cancer. Overall, our method represents an improved strategy for studying interstitial flows and interstitial transport in tumors and patients. We expect that our method will contribute to the better understanding of cancer progression and therapeutic response.
© 2024 Author(s).
Conflict of interest statement
The methodologies described herein are disclosed and claimed in a pending patent application co-owned by City of Hope and Virginia Polytechnic Institute and State University listing R.T.W., J.J.C., R.C.R., and J.M.M. as co-inventors. R.T.W., J.J.C., C.A.S., R.C.R., and J.M.M. are co-founders of and hold equity in Cairina Inc.
Figures


Update of
-
Model discovery approach enables non-invasive measurement of intra-tumoral fluid transport in dynamic MRI.bioRxiv [Preprint]. 2023 Oct 16:2023.08.28.554919. doi: 10.1101/2023.08.28.554919. bioRxiv. 2023. Update in: APL Bioeng. 2024 Apr 29;8(2):026106. doi: 10.1063/5.0190561. PMID: 37693372 Free PMC article. Updated. Preprint.

