Performance evaluation of the Access HBsAg and Access HBsAg confirmatory assays on the DxI 9000 Access Immunoassay Analyzer
- PMID: 38715659
- PMCID: PMC11075052
- DOI: 10.1016/j.plabm.2024.e00390
Performance evaluation of the Access HBsAg and Access HBsAg confirmatory assays on the DxI 9000 Access Immunoassay Analyzer
Abstract
Introduction: This study evaluated the clinical and analytical performances of the Access HBsAg and the Access HBsAg Confirmatory assays on the DxI 9000 Access Immunoassay Analyzer (Beckman Coulter, Inc.).
Materials and methods: Diagnostic specificity and sensitivity of the Access HBsAg and Access HBsAg Confirmatory assays were evaluated by comparing the Access assays to the final HBsAg sample status determined using the Architect, PRISM, or Elecsys HBsAg assays, along with Architect or PRISM HBsAg Confirmatory assays. Imprecision, sensitivity on seroconversion panels, analytical sensitivity on WHO, and recognition of HBV variants were also evaluated.
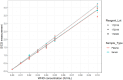
Results: A total of 7534 samples were included in the analysis (6047 blood donors, 1032 hospitalized patients, 455 positive patients' samples). Access HBsAg assay sensitivity and specificity were at 100.00% (99.19-100.0) and 99.92% (99.82-99.97), respectively. Sensitivity of Access HBsAg Confirmatory assay was 100.00% (99.21-100.0) on the 464 HBsAg positive samples. The use of a high positive algorithm for the Access HBsAg assay, wherein samples with S/CO ≥ 100.00 were considered positive without requiring repeat or confirmatory testing, was successfully evaluated with all 450 specimens with S/CO greater than 100.00 (sensitivity 100.00%; 99.19-100.0). Access HBsAg assay demonstrated good analytical performance, equivalent recognition of seroconversion panels compared to Architect assay, and an analytical sensitivity between 0.022 and 0.025 IU/mL. All HBV genotypes, subtypes and mutants were well detected without analytical sensitivity loss.
Conclusion: Access HBsAg and Access HBsAg Confirmatory assays demonstrated robust performances. They provide low samples volume requirements and a simplified process, no systematic retesting for high positive samples.
Keywords: Analytical performances; Chemiluminescence immunoassay; Clinical performances; HBsAg; Hepatitis B infection.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
B.V., J.G., F.B., C.C., C.V., S.G., I.V., V.L., J-C.P., Y-E.H., E.B. declare no conflicts of interest, none of them received personal fees from Beckman Coulter and all are employees of institutions or companies paid by Beckman Coulter to perform this study. V.R., J.H., S.B. and M.T. are employees of Beckman Coulter.
Figures
References
-
- Hepatitis B n.d. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed April 25, 2023).
LinkOut - more resources
Full Text Sources