Management advances for congenital diaphragmatic hernia: integrating prenatal and postnatal perspectives
- PMID: 38715680
- PMCID: PMC11071032
- DOI: 10.21037/tp-23-602
Management advances for congenital diaphragmatic hernia: integrating prenatal and postnatal perspectives
Abstract
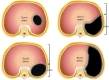
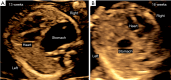
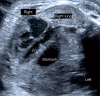
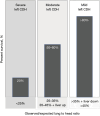
In congenital diaphragmatic hernia (CDH), abdominal organs are displaced into the chest, compress the lungs, and cause mediastinal shift. This contributes to development of pulmonary hypoplasia and hypertension, which is the primary determinant of morbidity and mortality for affected newborns. The severity is determined using prenatal imaging as early as the first trimester and is related to the laterality of the defect, extent of lung compression, and degree of liver herniation. Comprehensive evaluation of fetal CDH includes imaging-based severity assessment, severity assessment, and evaluation for structural or genetic abnormalities to differentiate isolated from complex cases. Prenatal management involves multispecialty counseling, consideration for fetal therapy with fetoscopic endoluminal tracheal occlusion (FETO) for severe cases, monitoring and intervention for associated polyhydramnios or signs of preterm labor if indicated, administration of antenatal corticosteroids in the appropriate setting, and planned delivery to optimize the fetal condition at birth. Integrated programs that provide a smooth transition from prenatal to postnatal care produce better outcomes. Neonatal care involves gentle ventilation to avoid hyperinflation and must account for transitional physiology to avoid exacerbating cardiac dysfunction and decompensation. Infants who have undergone and responded to FETO have greater pulmonary capacity than expected, but cardiac dysfunction seems unaffected. In about 25-30% of CDH neonates extracorporeal life support is utilized, and this provides a survival benefit for patients with the highest predicted mortality, including those who underwent FETO. Surgical repair after initial medical management for the first 24-48 hours of life is preferred since later repair is associated with delayed oral feeding, increased need for tube feeds, and increased post-repair ventilation requirement and supplemental oxygen at discharge. With overall survival rates >70%, contemporary care involves management of chronic morbidities in the context of a multidisciplinary clinic setting.
Keywords: Congenital diaphragmatic hernia (CDH); extracorporeal life support; fetoscopic endoluminal tracheal occlusion (FETO); gentle ventilation; surgical repair.
2024 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-602/coif). A.A.B. receives royalties from UpToDate and in-kind donation of fetoscopy sheath equipment from Karl Storz. J.L.M. is supported by Janssen Research and Development, LLC; serves as a Data Safety Monitoring Board member for FETO, and on the Executive Board of the North American Fetal Therapy Network. The other authors have no conflicts of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
