The brain-specific kinase LMTK3 regulates neuronal excitability by decreasing KCC2-dependent neuronal Cl- extrusion
- PMID: 38715938
- PMCID: PMC11075064
- DOI: 10.1016/j.isci.2024.109512
The brain-specific kinase LMTK3 regulates neuronal excitability by decreasing KCC2-dependent neuronal Cl- extrusion
Abstract
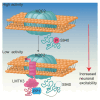
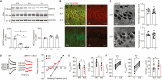
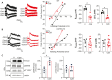
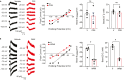
LMTK3 is a brain-specific transmembrane serine/threonine protein kinase that acts as a scaffold for protein phosphatase-1 (PP1). Although LMKT3 has been identified as a risk factor for autism and epilepsy, its physiological significance is unknown. Here, we demonstrate that LMTK3 copurifies and binds to KCC2, a neuron-specific K+/Cl- transporter. KCC2 activity is essential for Cl--mediated hyperpolarizing GABAAR receptor currents, the unitary events that underpin fast synaptic inhibition. LMTK3 acts to promote the association of KCC2 with PP1 to promote the dephosphorylation of S940 within its C-terminal cytoplasmic domain, a process the diminishes KCC2 activity. Accordingly, acute inhibition of LMTK3 increases KCC2 activity dependent upon S940 and increases neuronal Cl- extrusion. Consistent with this, LMTK3 inhibition reduced intrinsic neuronal excitability and the severity of seizure-like events in vitro. Thus, LMTK3 may have profound effects on neuronal excitability as an endogenous modulator of KCC2 activity.
Keywords: Biochemistry; Molecular biology; Neuroscience; Omics; Proteomics.
Crown Copyright © 2024 Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

