American Society of Pain and Neuroscience Best Practice (ASPN) Guideline for the Treatment of Sacroiliac Disorders
- PMID: 38716038
- PMCID: PMC11075694
- DOI: 10.2147/JPR.S464393
American Society of Pain and Neuroscience Best Practice (ASPN) Guideline for the Treatment of Sacroiliac Disorders
Abstract
Clinical management of sacroiliac disease has proven challenging from both diagnostic and therapeutic perspectives. Although it is widely regarded as a common source of low back pain, little consensus exists on the appropriate clinical management of sacroiliac joint pain and dysfunction. Understanding the biomechanics, innervation, and function of this complex load bearing joint is critical to formulating appropriate treatment algorithms for SI joint disorders. ASPN has developed this comprehensive practice guideline to serve as a foundational reference on the appropriate management of SI joint disorders utilizing the best available evidence and serve as a foundational guide for the treatment of adult patients in the United States and globally.
Keywords: best practices; chronic pain; radiofrequency ablation; sacroiliac joint; sacroiliac joint fusion; sacroiliitis.
© 2024 Sayed et al.
Conflict of interest statement
All authors were required to disclose conflicts of interest prior to assignment of topics. The senior authors determined the extent of the conflict of interest ensuring balanced inquiry and evaluation for each manuscript section. One of the co-primary authors without conflict were identified for each section and was the adjudication determination official for any issues of potential conflict. All authors were asked to recuse themselves on any recommendation potentially affected by a disclosed conflict. Additionally, authors without conflict vetted all recommendations for bias.DS is a consultant to Abbott, PainTEQ, Saluda, Mainstay, Surgentec, Nevro, and holds stock options with PainTEQ, Neuralace, Mainstay, Vertos, and SPR. TRD is a consultant PainTEQ, CornerLoc, and Spinal Simplicity. VTF receives research funding from Nevro Corporation part of an investigator-initiated study grant that is not related to this manuscript. TEW is a consultant for Medtronic and has received research funding from Medtronic, SPR Therapeutics, Nevro, and Boston Scientific. JSW is a consultant for Abbott, SI Bone, Vertos Medical, Biotronik, Saluda, and AbbVie, receives research funding from Abbott, SI Bone, Saluda, and Medtronic, serves on an advisory board for Abbott, SI Bone, Vertos Medical, and Biotronik, and serves on a speaker board for Abbott, SI Bone, & Abbvie. RSD receives investigator-initiated research grant funding from Nevro Corp and Saol Therapeutics that is paid to his institution. KA is a consultant for Nevro, Saluda, Biotronik, Boston Scientific, and Presidio, reports minor options from PainTEQ. MJD is a consultant for Globus, Camber, LifeSpine, Vyrsa, PainTEQ, Nevro, Abbott, and Biotronik. DTDN is a consultant for SI Bone, Stratus Medical, and Neurovassis. CB is a consultant for Nevro, Abbott, Vertos, Spinal Simplicity, and PainTEQ; reports personal fees from Nevro, Spinal Simplicity, PainTEQ, and Boston Scientific, outside the submitted work. AA is a consultant for Curonix, Medtronic and Avanos. The authors report no other conflicts of interest in this work.
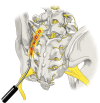
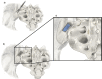
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

