Immune responses associated with protection induced by chemoattenuated PfSPZ vaccine in malaria-naive Europeans
- PMID: 38716733
- PMCID: PMC11141902
- DOI: 10.1172/jci.insight.170210
Immune responses associated with protection induced by chemoattenuated PfSPZ vaccine in malaria-naive Europeans
Abstract
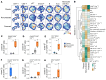
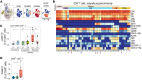
Vaccination of malaria-naive volunteers with a high dose of Plasmodium falciparum sporozoites chemoattenuated by chloroquine (CQ) (PfSPZ-CVac [CQ]) has previously demonstrated full protection against controlled human malaria infection (CHMI). However, lower doses of PfSPZ-CVac [CQ] resulted in incomplete protection. This provides the opportunity to understand the immune mechanisms needed for better vaccine-induced protection by comparing individuals who were protected with those not protected. Using mass cytometry, we characterized immune cell composition and responses of malaria-naive European volunteers who received either lower doses of PfSPZ-CVac [CQ], resulting in 50% protection irrespective of the dose, or a placebo vaccination, with everyone becoming infected following CHMI. Clusters of CD4+ and γδ T cells associated with protection were identified, consistent with their known role in malaria immunity. Additionally, EMRA CD8+ T cells and CD56+CD8+ T cell clusters were associated with protection. In a cohort from a malaria-endemic area in Gabon, these CD8+ T cell clusters were also associated with parasitemia control in individuals with lifelong exposure to malaria. Upon stimulation with P. falciparum-infected erythrocytes, CD4+, γδ, and EMRA CD8+ T cells produced IFN-γ and/or TNF, indicating their ability to mediate responses that eliminate malaria parasites.
Keywords: Cellular immune response; Cytokines; Immunology; Malaria; Vaccines.
Conflict of interest statement
Figures



References
-
- WHO. World Malaria Report 2023. https://www.who.int/publications/i/item/9789240086173 Accessed March 18, 2024.
-
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

