A Multicenter Randomized Controlled Trial of Microbiome-Based Artificial Intelligence-Assisted Personalized Diet vs Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet: A Novel Approach for the Management of Irritable Bowel Syndrome
- PMID: 38717025
- PMCID: PMC11365594
- DOI: 10.14309/ajg.0000000000002862
A Multicenter Randomized Controlled Trial of Microbiome-Based Artificial Intelligence-Assisted Personalized Diet vs Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet: A Novel Approach for the Management of Irritable Bowel Syndrome
Abstract
Introduction: Personalized management strategies are pivotal in addressing irritable bowel syndrome (IBS). This multicenter randomized controlled trial focuses on comparing the efficacy of a microbiome-based artificial intelligence-assisted personalized diet (PD) with a low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet (FODMAP) for IBS management.
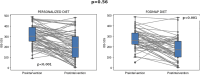
Methods: One hundred twenty-one patients participated, with 70 assigned to the PD group and 51 to the FODMAP diet group. IBS subtypes, demographics, symptom severity (IBS-SSS), anxiety, depression, and quality of life (IBS-QOL) were evaluated. Both interventions spanned 6 weeks. The trial's primary outcome was the within-individual difference in IBS-SSS compared between intervention groups.
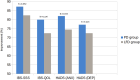
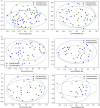
Results: For the primary outcome, there was a change in IBS-SSS of -112.7 for those in the PD group vs -99.9 for those in the FODMAP diet group ( P = 0.29). Significant improvement occurred in IBS-SSS scores ( P < 0.001), frequency ( P < 0.001), abdominal distension ( P < 0.001), and life interference ( P < 0.001) in both groups. In addition, there were significant improvements in anxiety levels and IBS-QOL scores for both groups ( P < 0.001). Importantly, PD was effective in reducing IBS SSS scores across all IBS subtypes IBS-Constipation (IBS-C; P < 0.001), IBS-Diarrhea (IBS-D; P = 0.01), and IBS-Mixed (IBS-M; P < 0.001) while FODMAP diet exhibited comparable improvements in IBS-C ( P = 0.004) and IBS-M ( P < 0.001). PD intervention significantly improved IBS-QOL scores for all subtypes (IBS-C [ P < 0.001], IBS-D [ P < 0.001], and IBS-M [ P = 0.008]) while the FODMAP diet did so for the IBS-C ( P = 0.004) and IBS-D ( P = 0.022). Notably, PD intervention led to significant microbiome diversity shifts ( P < 0.05) and taxa alterations compared with FODMAP diet.
Discussion: The artificial intelligence-assisted PD emerges as a promising approach for comprehensive IBS management. With its ability to address individual variation, the PD approach demonstrates significant symptom relief, enhanced QOL, and notable diversity shifts in the gut microbiome, making it a valuable strategy in the evolving landscape of IBS care.
Trial registration: ClinicalTrials.gov NCT05646186.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



References
-
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313(9):949–58. - PubMed
-
- Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 2021;160(1):99–114.e3. - PubMed
-
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012;10(7):712–21.e4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

