Molecular Landscape and Clinical Implication of CCNE1-amplified Esophagogastric Cancer
- PMID: 38717153
- PMCID: PMC11146286
- DOI: 10.1158/2767-9764.CRC-23-0496
Molecular Landscape and Clinical Implication of CCNE1-amplified Esophagogastric Cancer
Abstract
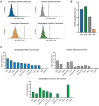
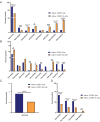
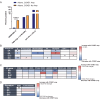
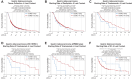
Cyclin E overexpression as a result of CCNE1 amplification is a critical driver of genomic instability in gastric cancer, but its clinical implication is largely unknown. Thus, we integrated genomic, transcriptomic, and immune profiling analysis of 7,083 esophagogastric tumors and investigated the impact of CCNE1 amplification on molecular features and treatment outcomes. We identified CCNE1 amplification in 6.2% of esophageal adenocarcinoma samples, 7.0% of esophagogastric junction carcinoma, 4.2% of gastric adenocarcinoma samples, and 0.8% of esophageal squamous cell carcinoma. Metastatic sites such as lymph node and liver showed an increased frequency of CCNE1 amplification relative to primary tumors. Consistent with a chromosomal instability phenotype, CCNE1 amplification was associated with decreased CDH1 mutation and increased TP53 mutation and ERBB2 amplification. We observed no differences in immune biomarkers such as PD-L1 expression and tumor mutational burden comparing CCNE1-amplified and nonamplified tumors, although CCNE1 amplification was associated with changes in immune populations such as decreased B cells and increased M1 macrophages from transcriptional analysis. Real-world survival analysis demonstrated that patients with CCNE1-amplified gastric cancer had worse survival after trastuzumab for HER2-positive tumors, but better survival after immunotherapy. These data suggest that CCNE1-amplified gastric cancer has a distinct molecular and immune profile with important therapeutic implications, and therefore further investigation of CCNE1 amplification as a predictive biomarker is warranted.
Significance: Advanced gastric cancer has a relatively dismal outcome with a 5-year overall survival of less than 10%. Furthermore, while comprehensive molecular analyses have established molecular subtypes within gastric cancers, biomarkers of clinical relevance in this cancer type are lacking. Overall, this study demonstrates that CCNE1 amplification is associated with a distinct molecular profile in gastric cancer and may impact response to therapy, including targeted therapy and/or immunotherapy.
© 2024 The Authors; Published by the American Association for Cancer Research.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet 2020;396:635–48. - PubMed
-
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. . First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

