Gliomatosis cerebri in children: A poor prognostic phenotype of diffuse gliomas with a distinct molecular profile
- PMID: 38717379
- PMCID: PMC11376460
- DOI: 10.1093/neuonc/noae080
Gliomatosis cerebri in children: A poor prognostic phenotype of diffuse gliomas with a distinct molecular profile
Abstract
Background: The term gliomatosis cerebri (GC), a radiology-defined highly infiltrating diffuse glioma, has been abandoned since molecular GC-associated features could not be established.
Methods: We conducted a multinational retrospective study of 104 children and adolescents with GC providing comprehensive clinical and (epi-)genetic characterization.
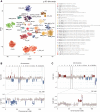
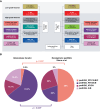
Results: Median overall survival (OS) was 15.5 months (interquartile range, 10.9-27.7) with a 2-year survival rate of 28%. Histopathological grading correlated significantly with median OS: CNS WHO grade II: 47.8 months (25.2-55.7); grade III: 15.9 months (11.4-26.3); grade IV: 10.4 months (8.8-14.4). By DNA methylation profiling (n = 49), most tumors were classified as pediatric-type diffuse high-grade glioma (pedHGG), H3-/IDH-wild-type (n = 31/49, 63.3%) with enriched subclasses pedHGG_RTK2 (n = 19), pedHGG_A/B (n = 6), and pedHGG_MYCN (n = 5), but only one pedHGG_RTK1 case. Within the pedHGG, H3-/IDH-wild-type subgroup, recurrent alterations in EGFR (n = 10) and BCOR (n = 9) were identified. Additionally, we observed structural aberrations in chromosome 6 in 16/49 tumors (32.7%) across tumor types. In the pedHGG, H3-/IDH-wild-type subgroup TP53 alterations had a significant negative effect on OS.
Conclusions: Contrary to previous studies, our representative pediatric GC study provides evidence that GC has a strong predilection to arise on the background of specific molecular features (especially pedHGG_RTK2, pedHGG_A/B, EGFR and BCOR mutations, chromosome 6 rearrangements).
Keywords: H3-wild-type and IDH-wild-type; chromosome 6; gliomatosis cerebri; pedHGG_RTK2; pediatric-type glioma; pediatric-type high-grade glioma.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
D.T.W.J. and S.M.P. are shareholders and cofounders of Heidelberg Epignostix GmbH. All remaining authors have declared that they have no competing interests.
Figures




References
-
- Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- German- and Styrian Childhood Cancer Foundation
- Etoile de Martin
- Les Boucles du Cœur de la Fondation Carrefour
- BB-033-00065/Necker Imagine Tumor and DNA biobank
- Rudy A Menon Foundation
- CRIS Cancer Foundation
- Ollie Young Foundation
- C13468/A23536/CRUK_/Cancer Research UK/United Kingdom
- Joshua Bembo Project and the AYJ Fund
- NHS
- NIHR Great Ormond Street Hospital Biomedical Research Centre
- PRIMUS/19/MED/06/Izas, la Princesa Guisante Foundation
- Charles University Grant Agency
- Fondazione Heal
- Il Coraggio dei Bambini
- Martina e La Sua Luna
- Il laboratorio di Chiara
- NET-2019-12371188/Regione Lombardia
- Italian Ministry of Health
- NET-2019-12371188/Regione Liguria
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

