International Expert Consensus on Semantics of Multimodal Esophageal Cancer Treatment: Delphi Study
- PMID: 38717548
- PMCID: PMC11236823
- DOI: 10.1245/s10434-024-15367-w
International Expert Consensus on Semantics of Multimodal Esophageal Cancer Treatment: Delphi Study
Abstract
Background: Recent developments in esophageal cancer treatment, including studies exploring active surveillance following chemoradiotherapy, have led to a need for clear terminology and definitions regarding different multimodal treatment options.
Objective: The aim of this study was to reach worldwide consensus on the definitions and semantics of multimodal esophageal cancer treatment.
Methods: In total, 72 experts working in the field of multimodal esophageal cancer treatment were invited to participate in this Delphi study. The study comprised three Delphi surveys sent out by email and one online meeting. Input for the Delphi survey consisted of terminology obtained from a systematic literature search. Participants were asked to respond to open questions and to indicate whether they agreed or disagreed with different statements. Consensus was reached when there was ≥75% agreement among respondents.
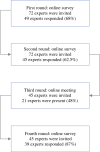
Results: Forty-nine of 72 invited experts (68.1%) participated in the first online Delphi survey, 45 (62.5%) in the second survey, 21 (46.7%) of 45 in the online meeting, and 39 (86.7%) of 45 in the final survey. Consensus on neoadjuvant and definitive chemoradiotherapy with or without surgery was reached for 27 of 31 items (87%). No consensus was reached on follow-up after treatment with definitive chemoradiotherapy.
Conclusion(s): Consensus was reached on most statements regarding terminology and definitions of multimodal esophageal cancer treatment. Implementing uniform criteria facilitates comparison of studies and promotes international research collaborations.
Keywords: Active surveillance; Definitive chemoradiotherapy; Delphi consensus study; Esophageal cancer; Expert consensus; Neoadjuvant chemoradiotherapy.
© 2024. The Author(s).
Conflict of interest statement
Bianca Mostert: Research funding from Sanofi, Pfizer and BMS; consulting/advisory for Lilly, Servier, BMS, Amgen and AstraZeneca. Bas Wijnhoven: Research funding from BMS; consulting/advisory for BMS. Grard Nieuwenhuijzen: Consulting/advisory for Medtronic, Lilly. Guillaume Piessen: Consulting/advisory for Nestle, BMS, MSD, Daiichi, Astellas. Ewout A. Kouwenhoven: Consulting/advisory for Intuitive Surgical. Ken Kato: Research funding from ONO, BMS, MSD; consulting/advisory for BMS, MSD, Beigene, Chugai, and BAYER. All remaining authors have declared no conflicts of interest in relation to this work.
Figures
References
-
- Eyck BM, van der Wilk BJ, Noordman BJ, et al. Updated protocol of the SANO trial: a stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials. 2021;22(1):345. doi: 10.1186/s13063-021-05274-w. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical