Prospective benchmarking of an observational analysis in the SWEDEHEART registry against the REDUCE-AMI randomized trial
- PMID: 38717556
- PMCID: PMC11101517
- DOI: 10.1007/s10654-024-01119-3
Prospective benchmarking of an observational analysis in the SWEDEHEART registry against the REDUCE-AMI randomized trial
Abstract
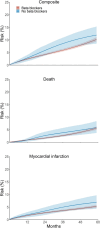
Prospective benchmarking of an observational analysis against a randomized trial increases confidence in the benchmarking process as it relies exclusively on aligning the protocol of the trial and the observational analysis, while the trials findings are unavailable. The Randomized Evaluation of Decreased Usage of Betablockers After Myocardial Infarction (REDUCE-AMI, ClinicalTrials.gov ID: NCT03278509) trial started recruitment in September 2017 and results are expected in 2024. REDUCE-AMI aimed to estimate the effect of long-term use of beta blockers on the risk of death and myocardial following a myocardial infarction with preserved left ventricular systolic ejection fraction. We specified the protocol of a target trial as similar as possible to that of REDUCE-AMI, then emulated the target trial using observational data from Swedish healthcare registries. Had everyone followed the treatment strategy as specified in the target trial protocol, the observational analysis estimated a reduction in the 5-year risk of death or myocardial infarction of 0.8 percentage points for beta blockers compared with no beta blockers; effects ranging from an absolute reduction of 4.5 percentage points to an increase of 2.8 percentage points in the risk of death or myocardial infarction were compatible with our data under conventional statistical criteria. Once results of REDUCE-AMI are published, we will compare the results of our observational analysis against those from the trial. If this prospective benchmarking is successful, it supports the credibility of additional analyses using these observational data, which can rapidly deliver answers to questions that could not be answered by the initial trial. If benchmarking proves unsuccessful, we will conduct a "postmortem" analysis to identify the reasons for the discrepancy. Prospective benchmarking shifts the investigator focus away from an endeavour to use observational data to obtain similar results as a completed randomized trial, to a systematic attempt to align the design and analysis of the trial and the observational analysis.
Keywords: Benchmarking; Causal inference; Randomized trials; Target trials.
© 2024. The Author(s).
Conflict of interest statement
M.A.H. is a consultant for Cytel and ProPublica, and a member of the Scientific Advisory Board for ADIA Lab, Flatiron and Foundation Medicine. R.H. reports speaker fees from Bristol Myers Squibb/Pfizer. All other authors declare no competing interests.
Figures

References
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

