Comparative pharmacokinetic evaluation of nanoparticle-based vs. conventional pharmaceuticals containing statins in attenuating dyslipidaemia
- PMID: 38717707
- PMCID: PMC11449971
- DOI: 10.1007/s00210-024-03140-5
Comparative pharmacokinetic evaluation of nanoparticle-based vs. conventional pharmaceuticals containing statins in attenuating dyslipidaemia
Abstract
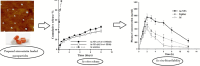
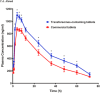
Dyslipidaemia describes the condition of abnormal lipid levels in a person's bloodstream. Since the 1980s, statin medications have been used to treat dyslipidaemia and other comorbidities, such as stroke risk and atherosclerosis. Statin medications were initially synthesised from fungal metabolites, but many synthetic statin drugs have been manufactured since then. Statin medication is quite effective in reducing total cholesterol levels in the bloodstream, but it has limitations. Due to their poor water solubility, statin drugs possess poor oral bioavailability, which hinders their therapeutic efficacy. Nanoparticle drug delivery technology has been shown to improve the pharmacokinetic profiles of many drug classes, and statins have great potential to benefit from this. This paper reviewed the currently available literature on nanoparticle statin medication and evaluated the possible improvements that can be made to the pharmacokinetic profile and efficacy of conventional statin medication. It was found that the oral bioavailability of nanoparticle medication consistently outperformed conventional medication by up to 400% in some cases. Substantial improvements in time to peak plasma concentration and plasma concentration peaks were also found, and increased periods in circulation before excretion were shown. It was concluded that nanoparticle technology has the potential to completely replace conventional statin medication as it offers more significant benefits with minimal drawbacks. Upon further study and development, the manufacture of nanoparticle statin medication should become feasible enough for large-scale application, which will significantly benefit patients and unburden healthcare systems.
Keywords: Drug delivery; Dyslipidaemia; Pharmacokinetics; Statin; Statin nanoparticles.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Ahmed TA (2021) Study the pharmacokinetics, pharmacodynamics and hepatoprotective activity of rosuvastatin from drug loaded lyophilized orodispersible tablets containing transfersomes nanoparticles. J Drug Deliv Sci Technol 63:102489. 10.1016/j.jddst.2021.102489
-
- Australian Bureau of Statistics (2013) Australian health survey: biomedical results for chronic diseases, 2011–12 | Australian Bureau of Statistics. https://www.abs.gov.au. https://www.abs.gov.au/statistics/health/health-conditions-and-risks/aus.... Accessed 24 Sept 2023
-
- Australian Institute of Health and Welfare (2017) Risk factors to health, Abnormal blood lipids (dyslipidaemia). https://www.aihw.gov.au/reports/risk-factors/risk-factors-to-health/cont.... Accessed 24 Sept 2023
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

