Endocytosis blocks the vesicular secretion of exosome marker proteins
- PMID: 38718108
- PMCID: PMC11078179
- DOI: 10.1126/sciadv.adi9156
Endocytosis blocks the vesicular secretion of exosome marker proteins
Abstract
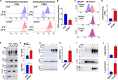
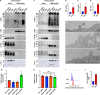
Exosomes are secreted vesicles of ~30 to 150 nm diameter that play important roles in human health and disease. To better understand how cells release these vesicles, we examined the biogenesis of the most highly enriched human exosome marker proteins, the exosomal tetraspanins CD81, CD9, and CD63. We show here that endocytosis inhibits their vesicular secretion and, in the case of CD9 and CD81, triggers their destruction. Furthermore, we show that syntenin, a previously described exosome biogenesis factor, drives the vesicular secretion of CD63 by blocking CD63 endocytosis and that other endocytosis inhibitors also induce the plasma membrane accumulation and vesicular secretion of CD63. Finally, we show that CD63 is an expression-dependent inhibitor of endocytosis that triggers the vesicular secretion of lysosomal proteins and the clathrin adaptor AP-2 mu2. These results suggest that the vesicular secretion of exosome marker proteins in exosome-sized vesicles occurs primarily by an endocytosis-independent pathway.
Figures





Update of
-
Syntenin and CD63 Promote Exosome Biogenesis from the Plasma Membrane by Blocking Cargo Endocytosis.bioRxiv [Preprint]. 2023 Jun 28:2023.05.26.542409. doi: 10.1101/2023.05.26.542409. bioRxiv. 2023. Update in: Sci Adv. 2024 May 10;10(19):eadi9156. doi: 10.1126/sciadv.adi9156. PMID: 37292617 Free PMC article. Updated. Preprint.
References
-
- Pegtel D. M., Gould S. J., Exosomes. Annu. Rev. Biochem. 88, 487–514 (2019). - PubMed
-
- Escola J. M., Kleijmeer M. J., Stoorvogel W., Griffith J. M., Yoshie O., Geuze H. J., Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273, 20121–20127 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

