Paxillin regulates androgen receptor expression associated with granulosa cell focal adhesions
- PMID: 38718206
- PMCID: PMC11136451
- DOI: 10.1093/molehr/gaae018
Paxillin regulates androgen receptor expression associated with granulosa cell focal adhesions
Abstract
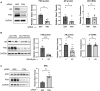
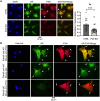
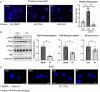
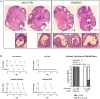
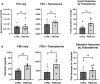
Paxillin is a ubiquitously expressed adaptor protein integral to focal adhesions, cell motility, and apoptosis. Paxillin has also recently been implicated as a mediator of nongenomic androgen receptor (AR) signaling in prostate cancer and other cells. We sought to investigate the relationship between paxillin and AR in granulosa cells (GCs), where androgen actions, apoptosis, and focal adhesions are of known importance, but where the role of paxillin is understudied. We recently showed that paxillin knockout in mouse GCs increases fertility in older mice. Here, we demonstrate that paxillin knockdown in human granulosa-derived KGN cells, as well as knockout in mouse primary GCs, results in reduced AR protein but not reduced mRNA expression. Further, we find that both AR protein and mRNA half-lives are reduced by approximately one-third in the absence of paxillin, but that cells adapt to chronic loss of paxillin by upregulating AR gene expression. Using co-immunofluorescence and proximity ligation assays, we show that paxillin and AR co-localize at the plasma membrane in GCs in a focal adhesion kinase-dependent way, and that disruption of focal adhesions leads to reduced AR protein level. Our findings suggest that paxillin recruits AR to the GC membrane, where it may be sequestered from proteasomal degradation and poised for nongenomic signaling, as reported in other tissues. To investigate the physiological significance of this in disorders of androgen excess, we tested the effect of GC-specific paxillin knockout in a mouse model of polycystic ovary syndrome (PCOS) induced by chronic postnatal dihydrotestosterone (DHT) exposure. While none of the control mice had estrous cycles, 33% of paxillin knockout mice were cycling, indicating that paxillin deletion may offer partial protection from the negative effects of androgen excess by reducing AR expression. Paxillin-knockout GCs from mice with DHT-induced PCOS also produced more estradiol than GCs from littermate controls. Thus, paxillin may be a novel target in the management of androgen-related disorders in women, such as PCOS.
Keywords: PCOS; androgen receptor; focal adhesion; granulosa; ovary; paxillin; polycystic ovary syndrome.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors disclose no conflicts of interest.
Figures


Similar articles
-
Paxillin knockout in mouse granulosa cells increases fecundity†.Biol Reprod. 2023 Nov 15;109(5):669-683. doi: 10.1093/biolre/ioad093. Biol Reprod. 2023. PMID: 37552051 Free PMC article.
-
Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome.Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):E3334-E3343. doi: 10.1073/pnas.1616467114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320971 Free PMC article.
-
N6-methyladenosine demethylase FTO related to hyperandrogenism in PCOS via AKT pathway.Gynecol Endocrinol. 2023 Oct 26;39(1):2276167. doi: 10.1080/09513590.2023.2276167. Epub 2023 Nov 6. Gynecol Endocrinol. 2023. PMID: 37931646
-
Androgen receptor-mediated non-genomic effects of vinclozolin on porcine ovarian follicles and isolated granulosa cells: Vinclozolin and non-genomic effects in porcine ovarian follicles.Acta Histochem. 2016 May;118(4):377-86. doi: 10.1016/j.acthis.2016.03.008. Epub 2016 Apr 16. Acta Histochem. 2016. PMID: 27094116
-
Physiological and Pathological Androgen Actions in the Ovary.Endocrinology. 2019 May 1;160(5):1166-1174. doi: 10.1210/en.2019-00101. Endocrinology. 2019. PMID: 30912811 Free PMC article. Review.
Cited by
-
Androgen Receptor PROTAC ARV-110 Ameliorates Metabolic Complications in a Mouse Model of Polycystic Ovary Syndrome.Endocrinology. 2025 May 19;166(7):bqaf091. doi: 10.1210/endocr/bqaf091. Endocrinology. 2025. PMID: 40437805
References
-
- Aflatounian A, Edwards MC, Rodriguez Paris V, Bertoldo MJ, Desai R, Gilchrist RB, Ledger WL, Handelsman DJ, Walters KA.. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. J Endocrinol 2020;245:381–395. - PubMed
-
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO.. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

