Synthesis and Regioselective Functionalization of Tetrafluorobenzo-[α]-Fused BOPYPY Dyes
- PMID: 38718291
- PMCID: PMC11110013
- DOI: 10.1021/acs.inorgchem.4c00499
Synthesis and Regioselective Functionalization of Tetrafluorobenzo-[α]-Fused BOPYPY Dyes
Abstract
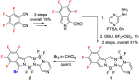
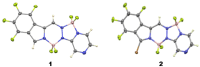
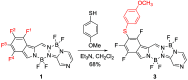
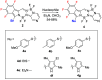
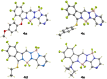
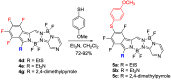
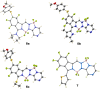
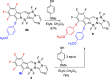
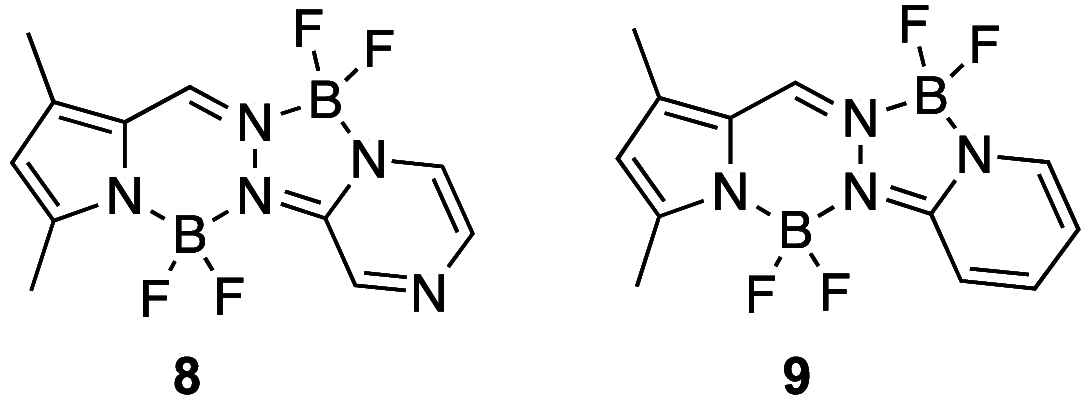
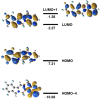
The synthesis of a new bis-BF2 tetrafluorobenzo-[α]-fused BOPYPY dye from 4,5,6,7-tetrafluoroisoindole and 2-hydrazinopyrazine is reported. The regioselectivity of nucleophilic substitution reactions at the periphery of the tetrafluorinated BOPYPY and its α-bromo derivative were investigated using N-, O-, S-, and C-based nucleophiles. Among the aromatic fluorine atoms, the F2 atom is consistently regioselectively substituted, except when the α-position contains a thiophenol group; in this case, F4 is substituted instead due to stabilizing π-π-stacking between the two aromatic groups. The α-bromo BOPYPY derivative also reacts under Stille cross-coupling reaction conditions to produce the corresponding α-substituted product. The spectroscopic properties of these new fluorinated BOPYPYs were investigated and compared with nonfluorinated analogs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

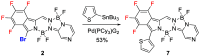
References
-
- Zhan X.; Kim D.; Ullah Z.; Lee W.; Gross Z.; Churchill D. G. Photophysics of Corroles and Closely Related Systems for Emergent Solar Energy, Medicinal, and Materials Science Applications. Coord. Chem. Rev. 2023, 495, 215363.10.1016/j.ccr.2023.215363. - DOI
-
- D’Alessandro S.; Priefer R. Non-Porphyrin Dyes Used as Photosensitizers in Photodynamic Therapy. J. Drug Delivery Sci. Technol. 2020, 60, 101979.10.1016/j.jddst.2020.101979. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

