Crosstalk between endothelial cells and dermal papilla entails hair regeneration and angiogenesis during aging
- PMID: 38718895
- PMCID: PMC11976415
- DOI: 10.1016/j.jare.2024.05.006
Crosstalk between endothelial cells and dermal papilla entails hair regeneration and angiogenesis during aging
Abstract
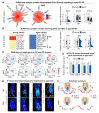
Introduction: Tissues maintain their function through interaction with microenvironment. During aging, both hair follicles and blood vessels (BV) in skin undergo degenerative changes. However, it is elusive whether the changes are due to intrinsic aging changes in hair follicles or blood vessels respectively, or their interactions.
Objective: To explore how hair follicles and blood vessels interact to regulate angiogenesis and hair regeneration during aging.
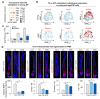
Methods: Single-cell RNA-sequencing (scRNA-seq) analyses were used to identify the declined ability of dermal papilla (DP) and endothelial cells (ECs) during aging. CellChat and CellCall were performed to investigate interaction between DP and ECs. Single-cell metabolism (scMetabolism) analysis and iPATH were applied to analyze downstream metabolites in DP and ECs. Hair-plucking model and mouse cell organoid model were used for functional studies.
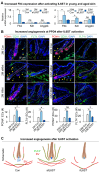
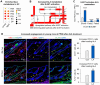
Results: During aging, distance and interaction between DP and ECs are decreased. DP interacts with ECs, with decreased EDN1-EDNRA signaling from ECs to DP and CTF1-IL6ST signaling from DP to ECs during aging. ECs-secreted EDN1 binds to DP-expressed EDNRA which enhances Taurine (TA) metabolism to promote hair regeneration. DP-emitted CTF1 binds to ECs-expressed IL6ST which activates alpha-linolenic acid (ALA) metabolism to promote angiogenesis. Activated EDN1-EDNRA-TA signaling promotes hair regeneration in aged mouse skin and in organoid cultures, and increased CTF1-IL6ST-ALA signaling also promotes angiogenesis in aged mouse skin and organoid cultures.
Conclusions: Our finding reveals reciprocal interactions between ECs and DP. ECs releases EDN1 sensed by DP to activate TA metabolism which induces hair regeneration, while DP emits CTF1 signal received by ECs to enhance ALA metabolism which promotes angiogenesis. Our study provides new insights into mutualistic cellular crosstalk between hair follicles and blood vessels, and identifies novel signaling contributing to the interactions of hair follicles and blood vessels in normal and aged skin.
Keywords: Aging; Angiogenesis; Cell interaction; Hair regeneration; Metabolism; Mutualism.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

