Zinc for prevention and treatment of the common cold
- PMID: 38719213
- PMCID: PMC11078591
- DOI: 10.1002/14651858.CD014914.pub2
Zinc for prevention and treatment of the common cold
Abstract
Background: The common cold is an acute, self-limiting viral respiratory illness. Symptoms include nasal congestion and mucus discharge, sneezing, sore throat, cough, and general malaise. Given the frequency of colds, they are a public health burden and a significant cause of lost work productivity and school absenteeism. There are no established interventions to prevent colds or shorten their duration. However, zinc supplements are commonly recommended and taken for this purpose.
Objectives: To assess the effectiveness and safety of zinc for the prevention and treatment of the common cold.
Search methods: We searched CENTRAL, MEDLINE, Embase, CINAHL, and LILACS to 22 May 2023, and searched Web of Science Core Collection and two trials registries to 14 June 2023. We also used reference checking, citation searching, and contact with study authors to identify additional studies.
Selection criteria: We included randomised controlled trials (RCTs) in children or adults that tested any form of zinc against placebo to prevent or treat the common cold or upper respiratory infection (URTI). We excluded zinc interventions in which zinc was combined with other minerals, vitamins, or herbs (e.g. a multivitamin, or mineral supplement containing zinc).
Data collection and analysis: We used the Cochrane risk of bias tool to assess risks of bias, and GRADE to assess the certainty of the evidence. We independently extracted data. When necessary, we contacted study authors for additional information. We assessed zinc (type and route) with placebo in the prevention and treatment of the common cold. Primary outcomes included the proportion of participants developing colds (for analyses of prevention trials only), duration of cold (measured in days from start to resolution of the cold), adverse events potentially due to zinc supplements (e.g. unpleasant taste, loss of smell, vomiting, stomach cramps, and diarrhoea), and adverse events considered to be potential complications of the common cold (e.g. respiratory bacterial infections).
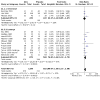
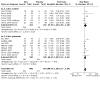
Main results: We included 34 studies (15 prevention, 19 treatment) involving 8526 participants. Twenty-two studies were conducted on adults and 12 studies were conducted on children. Most trials were conducted in the USA (n = 18), followed by India, Indonesia, Iran, and Turkey (two studies each), and Australia, Burkina Faso, Colombia, Denmark, Finland, Tanzania, Thailand, and the UK (one study each). The 15 prevention studies identified the condition as either common cold (n = 8) or URTI (n = 7). However, almost all therapeutic studies (17/19) focused on the common cold. Most studies (17/34) evaluated the effectiveness of zinc administered as lozenges (3 prevention; 14 treatment) in acetate, gluconate, and orotate forms; gluconate lozenges were the most common (9/17). Zinc gluconate was given at doses between 45 and 276 mg/day for between 4.5 and 21 days. Five (5/17) lozenge studies gave acetate lozenges and two (2/17) gave both acetate and gluconate lozenges. One (1/17) lozenge study administered intranasal (gluconate) and lozenge (orotate) zinc in tandem for cold treatment. Of the 17/34 studies that did not use lozenges, 1/17 gave capsules, 3/17 administered dissolved powders, 5/17 gave tablets, 4/17 used syrups, and 4/17 used intranasal administration. Most studies were at unclear or high risk of bias in at least one domain. There may be little or no reduction in the risk of developing a cold with zinc compared to placebo (risk ratio (RR) 0.93, 95% CI 0.85 to 1.01; I2 = 20%; 9 studies, 1449 participants; low-certainty evidence). There may be little or no reduction in the mean number of colds that occur over five to 18 months of follow-up (mean difference (MD) -0.90, 95% CI -1.93 to 0.12; I2 = 96%; 2 studies, 1284 participants; low-certainty evidence). When colds occur, there is probably little or no difference in the duration of colds in days (MD -0.63, 95% CI -1.29 to 0.04; I² = 77%; 3 studies, 740 participants; moderate-certainty evidence), and there may be little or no difference in global symptom severity (standardised mean difference (SMD) 0.04, 95% CI -0.35 to 0.43; I² = 0%; 2 studies, 101 participants; low-certainty evidence). When zinc is used for cold treatment, there may be a reduction in the mean duration of the cold in days (MD -2.37, 95% CI -4.21 to -0.53; I² = 97%; 8 studies, 972 participants; low-certainty evidence), although it is uncertain whether there is a reduction in the risk of having an ongoing cold at the end of follow-up (RR 0.52, 95% CI 0.21 to 1.27; I² = 65%; 5 studies, 357 participants; very low-certainty evidence), or global symptom severity (SMD -0.03, 95% CI -0.56 to 0.50; I² = 78%; 2 studies, 261 participants; very low-certainty evidence), and there may be little or no difference in the risk of a change in global symptom severity (RR 1.02, 95% CI 0.85 to 1.23; 1 study, 114 participants; low-certainty evidence). Thirty-one studies reported non-serious adverse events (2422 participants). It is uncertain whether there is a difference in the risk of adverse events with zinc used for cold prevention (RR 1.11, 95% CI 0.84 to 1.47; I2 = 0%; 7 studies, 1517 participants; very low-certainty evidence) or an increase in the risk of serious adverse events (RR 1.67, 95% CI 0.78 to 3.57; I2 = 0%; 3 studies, 1563 participants; low-certainty evidence). There is probably an increase in the risk of non-serious adverse events when zinc is used for cold treatment (RR 1.34, 95% CI 1.15 to 1.55; I2 = 44%; 2084 participants, 16 studies; moderate-certainty evidence); no treatment study provided information on serious adverse events. No study provided clear information about adverse events considered to be potential complications of the common cold.
Authors' conclusions: The findings suggest that zinc supplementation may have little or no effect on the prevention of colds but may reduce the duration of ongoing colds, with an increase in non-serious adverse events. Overall, there was wide variation in interventions (including concomitant therapy) and outcomes across the studies, as well as incomplete reporting of several domains, which should be considered when making conclusions about the efficacy of zinc for the common cold.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Daryl Nault: declares no conflict of interest.
Taryn Machingo: declares no conflict of interest.
Andrea G Shipper: declares no conflict of interest.
Daniel A Antiporta: declares no conflict of interest.
Candyce Hamel: declares no conflict of interest.
Sahar Nourouzpour: declares no conflict of interest.
Menelaos Konstantinidis: reports being a Statistical Editor for Cochrane Acute Respiratory Infections, but had no role in the editorial process of this review.
Erica Phillips: declares no conflict of interest.
Elizabeth Lipski: declares no conflict of interest.
L Susan Wieland: reports funding for Cochrane Complementary Medicine from the US National Institutes of Health, National Center for Complementary and Alternative Medicine, R24 AT001293; paid to institution.
Figures


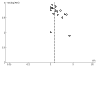
































































Update of
References
References to studies included in this review
Al‐Nakib 1987 {published data only}
Belongia 2001 {published data only}
Caesar 2012 {published data only}
-
- Caesar CS, Juffrie M, Sumadiono. Influence of zinc on severity of common cold in children. Paediatrica Indonesiana 2012;52(6):324-8. [DOI: 10.14238/pi52.6.2012.324-8] - DOI
Douglas 1987 {published data only}
Eby 1984 {published data only}
Eby 2006 {published data only}
-
- Eby GA, Halcomb WW. Ineffectiveness of zinc gluconate nasal spray and zinc orotate lozenges in common-cold treatment: a double-blind, placebo-controlled clinical trial. Alternative Therapies in Health & Medicine 2006;12(1):34-8. - PubMed
Farr Trial 1 1987 {published data only}
Farr Trial 2 1987 {published data only}
Godfrey 1992 {published data only}
Hemilä 2020 {published data only}
-
- Hemilä H. The effect of zinc acetate lozenges on the rate of recovery from the common cold. https://classic.clinicaltrials.gov/ct2/show/NCT03309995 (first received 16 October 2017). [CLINICALTRIALS.GOV: NCT03309995]
Hirt 2000 {published data only}
-
- Hirt M, Nobel S, Barron E. Zinc nasal gel for the treatment of common cold symptoms: a double-blind, placebo-controlled trial. Ear, Nose & Throat Journal 2000;79(10):778-80, 782. - PubMed
Kartasurya 2012 {published data only}
Kurugöl 2006 {published data only}
Kurugöl 2007 {published data only}
Macknin 1998 {published data only}
Malik 2014 {published data only}
Mandlik 2020 {published data only (unpublished sought but not used)}
-
- Mandlik R, Mughal Z, Khadilkar A, Chiplonkar S, Ekbote V, Kajale N, et al. Occurrence of infections in school children subsequent to supplementation with vitamin D-calcium or zinc: a randomized, double-blind, placebo-controlled trial. Nutrition Research and Practice 2020;14(2):117. [DOI: 10.4162/nrp.2020.14.2.117] - DOI - PMC - PubMed
McDonald 2015 {published and unpublished data}
-
- McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW, et al. Daily zinc but not multivitamin supplementation reduces diarrhea and upper respiratory infections in Tanzanian infants: a randomized, double-blind, placebo-controlled clinical trial. Journal of Nutrition 2015;145(9):2153-60. [DOI: 10.3945/jn.115.212308] - DOI - PMC - PubMed
Mossad 1996 {published data only}
Mossad 2003 {published data only}
Petrus 1998 {published data only}
-
- Petrus EJ, Lawson KA, Bucci LR, Blum K. Randomized, double-masked, placebo-controlled clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy- tested subjects. Current Therapeutic Research, Clinical and Experimental 1998;59(9):595-607. [DOI: 10.1016/S0011-393X(98)85058-3] - DOI - PMC - PubMed
Pooya 2006 {published data only}
-
- Pooya A, Mahmoudian A, Hazavei MM, Farajzadegan Z, Ramezani MA. The effect of zinc and "Health Belief Model" based education on common cold prevention in soldiers. American Journal of Infectious Diseases 2006;2(4):193-6. [DOI: 10.3844/ajidsp.2006.193.196] - DOI
Prasad 2000 {published data only}
-
- Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH, Prasad AS, et al. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine 2000;133(4):245-52. [DOI: 10.7326/0003-4819-133-4-200008150-00006] - DOI - PubMed
Prasad 2007 {published data only}
-
- Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. American Journal of Clinical Nutrition 2007;85(3):837-44. [DOI: 10.1093/ajcn/85.3.837] - DOI - PubMed
Prasad 2008 {published data only}
-
- Prasad AS, Beck FW, Bao B, Snell D, Fitzgerald JT. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. Journal of Infectious Diseases 2008;197(6):795-802. [DOI: 10.1086/528803] - DOI - PubMed
Rerksuppaphol 2013 {published data only}
Sánchez 2014 {published data only}
-
- Sánchez J, Villada OA, Rojas ML, Montoya L, Díaz A, Vargas C, et al. Effect of zinc amino acid chelate and zinc sulfate in the incidence of respiratory infection and diarrhoea in preschool children in children's centres [Efecto del zinc aminoquelado y el sulfato de zinc en la incidencia de la infección respiratoria y la diarrea en niños preescolares de centros infantiles^ies]. Biomédica (Bogotá) 2014;34(1):79-91. - PubMed
Smith 1989 {published data only}
Somé 2015 {published data only}
-
- Somé JW, Abbeddou S, Yakes Jimenez E, Hess SY, Ouédraogo ZP, Guissou RM, et al. Effect of zinc added to a daily small-quantity lipid-based nutrient supplement on diarrhoea, malaria, fever and respiratory infections in young children in rural Burkina Faso: a cluster-randomised trial. BMJ Open 2015;5(9):e007828. [DOI: 10.1136/bmjopen-2015-007828] - DOI - PMC - PubMed
Turner 2000 challenge {published data only}
Turner 2000 natural {published data only (unpublished sought but not used)}
Turner 2001 {published data only}
Vakili 2009 {published data only}
-
- Vakili R, Vahedian M, Khodaei G, Mahmoudi M. Effects of zinc supplementation in occurrence and duration of common cold in school aged children during cold season: a double-blind placebo-controlled trial. Iranian Journal of Pediatrics 2009;19(4):376-80.
Weismann 1990 {published data only}
-
- Weismann K, Jakobsen JP, Weismann JE, Hammer UM, Nyholm SM, Hansen B, et al. Zinc gluconate lozenges for common cold. A double-blind clinical trial. Danish Medical Bulletin 1990;37(3):279-81. - PubMed
References to studies excluded from this review
Abdulhamid 2008 {published data only}
Australian Journal of Pharmacy 2011 {published data only}
-
- Zinc reduces the duration and severity of the common cold in healthy people. Australian Journal of Pharmacy 2011;92(1093):86.
Bansal 2011 {published data only}
Barffour 2020 {published data only}
-
- Barffour MA, Hinnouho G-M, Wessells KR, Kounnavong S, Ratsavong K, Sitthideth D, et al. Effects of therapeutic zinc supplementation for diarrhea and two preventive zinc supplementation regimens on the incidence and duration of diarrhea and acute respiratory tract infections in rural Laotian children: a randomized controlled trial. Journal of Global Health 2020;10(1):010424. [DOI: 10.7189/jogh.10.010424] - DOI - PMC - PubMed
Bates 1993 {published data only}
Brown 2007 {published data only}
-
- Brown KH, López de Romaña D, Arsenault JE, Peerson JM, Penny ME. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. American Journal of Clinical Nutrition 2007;85(2):538-47. [DOI: 10.1093/ajcn/85.2.538] - DOI - PubMed
Chandyo 2010 {published data only}
-
- Chandyo RK, Shrestha PS, Valentiner-Branth P, Mathisen M, Basnet S, Ulak M, et al. Two weeks of zinc administration to Nepalese children with pneumonia does not reduce the incidence of pneumonia or diarrhea during the next six months. Journal of Nutrition 2010;140(9):1677-82. [DOI: 10.3945/jn.109.117978] - DOI - PubMed
Girard 1991 {published data only}
-
- Girard JP, Terki N. Zinc gluconate in the treatment of rhinitis due to graminaceous pollen. Revue Francaise d'Allergologie 1991;31(3):157-60.
Habibian 2013 {published data only}
-
- Habibian R, Khoshdel A, Kheiri S, Torabi A. The effect of zinc sulphate syrup on children’s respiratory tract infections [اثر شربت سولفات روی بر عالئم بالینی کودکان بستری مبتال به عفونت های تنفسی]. Journal of Babol University of Medical Sciences 2013;15(4):22-9.
-
- IRCT201103025951N1. Zinc effect on respiratory infections [Zinc sulfate effect on children with respiratory infection]. https://en.irct.ir/trial/6427 (first received 17 June 2011).
Khera 2020 {published data only}
Kujinga 2018 {published data only}
-
- Kujinga P, Galetti V, Onyango E, Jakab V, Buerkli S, Andang'o P, et al. Effectiveness of zinc-fortified water on zinc intake, status and morbidity in Kenyan pre-school children: a randomised controlled trial. Public Health Nutrition 2018;21(15):2855-65. [DOI: 10.1017/S1368980018001441] - DOI - PMC - PubMed
Lira 1998 {published data only}
Long 2006 {published data only}
-
- Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL. A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. American Journal of Clinical Nutrition 2006;83(3):693-700. [DOI: 10.1093/ajcn.83.3.693] - DOI - PubMed
Luabeya 2007 {published data only}
-
- Luabeya KK, Mpontshane N, Mackay M, Ward H, Elson I, Chhagan M, et al. Zinc or multiple micronutrient supplementation to reduce diarrhea and respiratory disease in South African children: a randomized controlled trial. PLOS One 2007;2(6):e541. [DOI: 10.1371/journal.pone.0000541] - DOI - PMC - PubMed
Maggini 2012 {published data only}
Makonnen 2003 {published data only}
-
- Makonnen B, Venter A, Joubert G. A randomized controlled study of the impact of dietary zinc supplementation in the management of children with protein-energy malnutrition in Lesotho. II: Special investigations. Journal of Tropical Pediatrics 2003;49(6):353-60. [DOI: 10.1093/tropej/49.6.353] - DOI - PubMed
Martinez‐Estevez 2016 {published data only}
-
- Martinez-Estevez NS, Alvarez-Guevara AN, Rodriguez-Martinez CE. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: a 12-month randomised controlled trial. Allergologia et Immunopathologia 2016;44(4):368-75. [DOI: 10.1016/j.aller.2015.12.006] - DOI - PubMed
Masoodpoor 2008 {published data only}
-
- Masoodpoor N, Darakhshan S, Darakhshan D, Tabatabaei ST, Mosavat SA. Impact of zinc supplementation on respiratory and gastrointestinal infections: a double-blind, randomized trial among urban Iranian school children. Pediatrics 2008;121(Suppl 2):S153-4.
McElroy 2003 {published data only}
-
- McElroy BH, Miller SP, McElroy BH, Miller SP. An open-label, single-center, phase IV clinical study of the effectiveness of zinc gluconate glycine lozenges (Cold-Eeze) in reducing the duration and symptoms of the common cold in school-aged subjects. American Journal of Therapeutics 2003;10(5):324-9. [DOI: 10.1097/00045391-200309000-00004] - DOI - PubMed
Meeks Gardner1998 {published data only}
NCT01092039 {published data only}
-
- NCT01092039. Safety and efficacy study of oral XIGO tablets to treat the common cold [XIGO effectiveness study: an investigation of the safety and efficacy of oral XIGO tablets on patients diagnosed with the common cold]. www.clinicaltrials.gov/study/NCT01092039 (first submitted 22 March 2010).
NCT04672850 {published data only}
-
- NCT04672850. The effect of probiotic and zinc supplementation on the common cold [The effect of probiotic and zinc supplementation on the common cold in healthy adults]. clinicaltrials.gov/ct2/show/NCT04672850 (first received 3 December 2020).
Ninh 1996 {published data only}
-
- Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. American Journal of Clinical Nutrition 1996;63(4):514-9. [DOI: 10.1093/ajcn/63.4.514] - DOI - PubMed
Penny 2004 {published data only}
-
- Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lönnerdal B, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. American Journal of Clinical Nutrition 2004;79(3):457-65. [DOI: 10.1093/ajcn/79.3.457] - DOI - PubMed
Rahman 2001 {published data only}
Richard 2006 {published data only}
-
- Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene 2006;75(1):126-32. [DOI: 10.4269/ajtmh.2006.75.1.0750126] - DOI - PubMed
Roy 1999 {published data only}
-
- Roy SK, Tomkins AM, Haider R, Behren RH, Akramuzzaman SM, Mahalanabis D, et al. Impact of zinc supplementation on subsequent growth and morbidity in Bangladeshi children with acute diarrhoea. European Journal of Clinical Nutrition 1999;53(7):529‐34. [DOI: DOI: 10.1038/sj.ejcn.1600734] - PubMed
Ruel 1997 {published data only}
Sampaio 2013 {published data only}
-
- Sampaio DL, De Mattos ÂP, Ribeiro TC, De Leite ME, Cole CR, Costa-Ribeiro H Jr. Zinc and other micronutrients supplementation through the use of sprinkles [Suplementação de zinco e outros micronutrientes através do uso de sprinkles: impacto na ocorrência de doença diarreica e infecções respiratórias em crianças institucionalizadas^ipt]. Jornal de Pediatria 2013;89(3):286-93. - PubMed
Sempértegui 1996 {published data only}
-
- Sempértegui F, Estrella B, Correa E, Aguirre L, Saa B, Torres M, et al. Effects of short-term zinc supplementation on cellular immunity, respiratory symptoms, and growth of malnourished Equadorian children. European Journal of Clinical Nutrition 1996;50(1):42-6. - PubMed
Shaker 2018 {published data only}
-
- Shaker SM, Fathy H, Abdelall EK, Said AS. The effect of zinc and Vitamin A supplements in treating and reducing the incidence of upper respiratory tract infections in children. National Journal of Physiology Pharmacy and Pharmacology 2018;8(7):1010-7. [DOI: 10.5455/njppp.2018.8.0104206032018] - DOI
Silk 2005 {published data only}
Smith 1991 {published data only}
-
- Smith AP, Tyrrell DA, Al-Nakib W, Barrow I, Higgins P, Wenham R. Effects of zinc gluconate and nedocromil sodium on performance deficits produced by the common cold. Journal of Psychopharmacology 1991;5(3):251-4. - PubMed
Taneja 2006 {published data only}
-
- Taneja S, Bhandari N, Rongsen-Chandola T, Mahalanabis D, Fontaine O, Bhan MK. Impact of zinc supplementation in low birth weight infants on severe morbidity, mortality and zinc status: a randomized controlled trial. https://www.clinicaltrials.gov/study/NCT00272142 (first received 3 January 2006).
Veverka 2009 {published data only}
Walker 2007 {published data only}
-
- Walker CL, Bhutta ZA, Bhandari N, Teka T, Shahid F, Taneja S, et al. Zinc during and in convalescence from diarrhea has no demonstrable effect on subsequent morbidity and anthropometric status among infants <6 mo of age. American Journal of Clinical Nutrition 2007;85(3):887-94. [DOI: 10.1093/ajcn/85.3.887] - DOI - PubMed
Yousefichaijan 2017 {published data only}
-
- IRCT138903184056N2. Effectiveness of zinc sulfate in common cold symptoms in children [Evaluation of the effectiveness of zinc sulfate in reducing duration of common cold symptoms in children]. https://www.irct.ir/trial/4235 (first received 14 June 2010).
-
- Yousefichaijan P, Dorreh F, Naziri M. The effect of zinc sulfate on duration of common cold symptoms in children. Journal of Biology and Today's World 2017;6(10):186-90.
References to studies awaiting assessment
Bobat 2005 {published data only}
-
- Bobat R, Coovadia H, Stephen C, Naidoo KL, McKerrow N, Black RE, et al. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: a randomised double-blind placebo-controlled trial. Lancet 2005;366(9500):1862-7. [DOI: 10.1016/S0140-6736(05)67756-2] - DOI - PubMed
NCT05002101 {published data only}
-
- NCT05002101. Daily zinc supplement effect on prevention of diarrhea and acute respiratory infections in children less than five years [The effect of daily zinc supplementation on prevention of diarrhea and acute respiratory infections among children less than five years: a randomized controlled trial]. clinicaltrials.gov/study/NCT05002101 (first received 1 August 2018).
Osendarp 2001 {published data only}
Wuehler 2008 {published data only}
Additional references
Atkins 2004
Caulfield 2004
-
- Caulfield LE, Black RE. Chapter 5: Zinc deficiency. In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Vol. 1. World Health Organization, 2004:257–80.
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
D'Cruze 2009
-
- D'Cruze H, Arroll B, Kenealy T. Is intranasal zinc effective and safe for the common cold? A systematic review and meta-analysis. Journal of Primary Health Care 2009;1(2):134-9. - PubMed
Deeks 2023
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Dicpinigaitis 2015
-
- Dicpinigaitis PV, Eccles R, Blaiss MS, Wingertzahn MA. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States: ACHOO Survey. Current Medical Research and Opinion 2015;31(8):1519-25. [PMID: ] - PubMed
Egger 1997
Harvard Health Letter 2014
-
- Harvard Health Letter. Could a cold remedy make you sicker? www.health.harvard.edu/staying-healthy/could-a-cold-remedy-make-you-sicker (accessed 1 March 2021).
Hemilä 2011
Hemilä 2015
Hemilä 2016
Hemilä 2017a
Hemilä 2017b
Higgins 2011
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. - PMC - PubMed
Hulisz 2004
Hunter 2021
-
- Hunter J, Arentz S, Goldenberg J, Yang G, Beardsley J, Myers SP, et al. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: a rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open 2021;11(11):e047474. [DOI: 10.1136/bmjopen-2020-047474] - DOI - PMC - PubMed
IOM 2001
IQWiG 2020
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Common colds: overview. www.ncbi.nlm.nih.gov/books/NBK279543/ (accessed 1 February 2021).
Jackson 1997
-
- Jackson JL, Peterson C, Lesho E. A meta-analysis of zinc salts lozenges and the common cold. Archives of Internal Medicine 1997;157(20):2373-6. - PubMed
Lefebvre 2023
-
- Lefebvre C, Glanville J, Briscoe S, Featherston R, LIttlewood A, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated October 2023). Cochrane, 2023. Available from training.cochrane.org/handbook.
Maares 2016
-
- Maares M, Haase H. Zinc and immunity: an essential interrelation. Archives of Biochemistry and Biophysics 2016;611:58-65. [PMID: ] - PubMed
Maares 2020
Marshall 2007
Moher 2009
Moshtagh 2020
Mäkelä 1998
Prasad 2020
Read 2019
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rink 2000
-
- Rink L, Gabriel P. Zinc and the immune system. Proceedings of the Nutrition Society 2000;59(4):541-52. [PMID: ] - PubMed
Science 2012
Singh 2015
Tabatabaeizadeh 2022
Wang 2020
Witek 2015
-
- Witek TJ, Ramsey DL, Carr AN, Riker DK. The natural history of community-acquired common colds symptoms assessed over 4-years. Rhinology 2015;53(1):81-8. [PMID: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

