Real-World Effectiveness of Pegloticase Associated With Use of Concomitant Immunomodulatory Therapy
- PMID: 38719773
- PMCID: PMC11424266
- DOI: 10.1002/acr.25361
Real-World Effectiveness of Pegloticase Associated With Use of Concomitant Immunomodulatory Therapy
Abstract
Objective: The objective of this study was to ascertain pegloticase persistence and adverse events associated with concomitant immunomodulatory drug treatment in patients with gout.
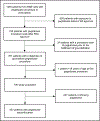
Methods: We conducted a retrospective analysis of patients with gout using the American College of Rheumatology's Rheumatology Informatics System for Effectiveness registry from January 2016 through June 2020. The first pegloticase infusion defined the index date. Based on concomitant immunomodulatory drug treatment, we identified three exposure groups: (1) immunomodulatory drug initiators (patients initiating an immunomodulatory prescription ±60 days from the index date), (2) prevalent immunomodulatory drug recipients (patients receiving their first immunomodulatory drug prescription >60 days before the index date with at least one prescription within ±60 days of the index date), and (3) immunomodulatory nonrecipients (patients receiving pegloticase without concomitant immunomodulatory drugs). We calculated the proportion of patients who achieved serum urate levels ≤6 mg/dL and who had laboratory abnormalities (white blood cell count <3.4 x 109/L, platelet count <135,000, hematocrit level <30%, alanine aminotransferase or aspartate aminotransferase level ≥1.5 times the upper limit normal value) within 180 days after the index date. Cox regression analyzed time to pegloticase discontinuation, controlling for potential confounders.
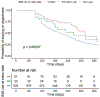
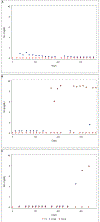
Results: We identified 700 pegloticase recipients (91 immunomodulatory drug initiators, 33 prevalent immunomodulatory drug recipients, and 576 nonrecipients), with a median follow-up of 14 months. Immunomodulatory drug recipients were less likely to discontinue pegloticase. The adjusted hazard ratios of pegloticase discontinuation associated with concomitant immunomodulatory drug initiation and prevalent treatment were 0.52 (95% confidence interval [CI] 0.37-0.75) and 0.69 (95% CI 0.42-1.16), respectively. Laboratory abnormalities were uncommon (<5%) and were not higher in concomitant immunomodulatory drug treatment.
Conclusion: Consistent with clinical trials, results from this large observational registry suggest that concomitant immunomodulatory drug treatment improves pegloticase persistence.
© 2024 The Authors. Arthritis Care & Research published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Safiri S, Kolahi A-A, Cross M, et al. Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990–2017: A systematic analysis of the global burden of disease study 2017. Arthritis Rheumatol. 2020;72(11):1916–1927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

