Bacteroidota inhibit microglia clearance of amyloid-beta and promote plaque deposition in Alzheimer's disease mouse models
- PMID: 38719797
- PMCID: PMC11078963
- DOI: 10.1038/s41467-024-47683-w
Bacteroidota inhibit microglia clearance of amyloid-beta and promote plaque deposition in Alzheimer's disease mouse models
Abstract
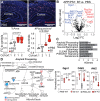
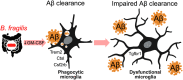
The gut microbiota and microglia play critical roles in Alzheimer's disease (AD), and elevated Bacteroides is correlated with cerebrospinal fluid amyloid-β (Aβ) and tau levels in AD. We hypothesize that Bacteroides contributes to AD by modulating microglia. Here we show that administering Bacteroides fragilis to APP/PS1-21 mice increases Aβ plaques in females, modulates cortical amyloid processing gene expression, and down regulates phagocytosis and protein degradation microglial gene expression. We further show that administering Bacteroides fragilis to aged wild-type male and female mice suppresses microglial uptake of Aβ1-42 injected into the hippocampus. Depleting murine Bacteroidota with metronidazole decreases amyloid load in aged 5xFAD mice, and activates microglial pathways related to phagocytosis, cytokine signaling, and lysosomal degradation. Taken together, our study demonstrates that members of the Bacteroidota phylum contribute to AD pathogenesis by suppressing microglia phagocytic function, which leads to impaired Aβ clearance and accumulation of amyloid plaques.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

