Unique lipid composition maintained by extracellular blockade leads to prooncogenicity
- PMID: 38719806
- PMCID: PMC11079073
- DOI: 10.1038/s41420-024-01971-y
Unique lipid composition maintained by extracellular blockade leads to prooncogenicity
Abstract
Lipid-mediated inflammation is involved in the development and malignancy of cancer. We previously demonstrated the existence of a novel oncogenic mechanism utilizing membrane lipids of extracellular vesicles in Epstein-Barr virus (EBV)-positive lymphomas and found that the lipid composition of lymphoma cells is skewed toward ω-3 fatty acids, which are anti-inflammatory lipids, suggesting an alteration in systemic lipid composition. The results showed that arachidonic acid (AA), an inflammatory lipid, was significantly reduced in the infected cells but detected at high levels in the sera of EBV-positive patients lead to the finding of the blockade of extracellular AA influx by downregulating FATP2, a long-chain fatty acid transporter that mainly transports AA in EBV-infected lymphoma cells. Low AA levels in tumor cells induced by downregulation of FATP2 expression confer resistance to ferroptosis and support tumor growth. TCGA data analysis and xenograft models have demonstrated that the axis plays a critical role in several types of cancers, especially poor prognostic cancers, such as glioblastoma and melanoma. Overall, our in vitro, in vivo, in silico, and clinical data suggest that several cancers exert oncogenic activity by maintaining their special lipid composition via extracellular blockade.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
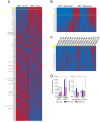
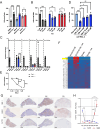

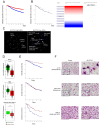
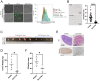
References
Grants and funding
- JP20H03716/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22K20838/MEXT | Japan Society for the Promotion of Science (JSPS)
- 2021-B04/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP19K07042/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22K06577/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources

