RAD18 O-GlcNAcylation promotes translesion DNA synthesis and homologous recombination repair
- PMID: 38719812
- PMCID: PMC11078974
- DOI: 10.1038/s41419-024-06700-y
RAD18 O-GlcNAcylation promotes translesion DNA synthesis and homologous recombination repair
Abstract
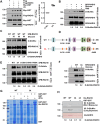
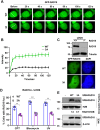
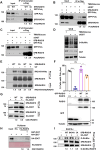
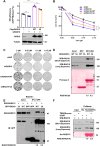
RAD18, an important ubiquitin E3 ligase, plays a dual role in translesion DNA synthesis (TLS) and homologous recombination (HR) repair. However, whether and how the regulatory mechanism of O-linked N-acetylglucosamine (O-GlcNAc) modification governing RAD18 and its function during these processes remains unknown. Here, we report that human RAD18, can undergo O-GlcNAcylation at Ser130/Ser164/Thr468, which is important for optimal RAD18 accumulation at DNA damage sites. Mechanistically, abrogation of RAD18 O-GlcNAcylation limits CDC7-dependent RAD18 Ser434 phosphorylation, which in turn significantly reduces damage-induced PCNA monoubiquitination, impairs Polη focus formation and enhances UV sensitivity. Moreover, the ubiquitin and RAD51C binding ability of RAD18 at DNA double-strand breaks (DSBs) is O-GlcNAcylation-dependent. O-GlcNAcylated RAD18 promotes the binding of RAD51 to damaged DNA during HR and decreases CPT hypersensitivity. Our findings demonstrate a novel role of RAD18 O-GlcNAcylation in TLS and HR regulation, establishing a new rationale to improve chemotherapeutic treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Kobayashi S, Kasaishi Y, Nakada S, Takagi T, Era S, Motegi A, et al. Rad18 and Rnf8 facilitate homologous recombination by two distinct mechanisms, promoting Rad51 focus formation and suppressing the toxic effect of nonhomologous end joining. Oncogene. 2015;34:4403–11. doi: 10.1038/onc.2014.371. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

