Periodic heat waves-induced neuronal etiology in the elderly is mediated by gut-liver-brain axis: a transcriptome profiling approach
- PMID: 38719902
- PMCID: PMC11079080
- DOI: 10.1038/s41598-024-60664-9
Periodic heat waves-induced neuronal etiology in the elderly is mediated by gut-liver-brain axis: a transcriptome profiling approach
Abstract
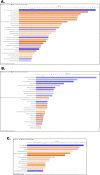
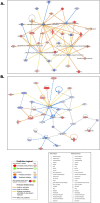
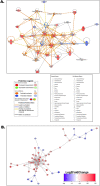
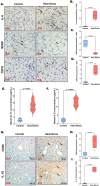
Heat stress exposure in intermittent heat waves and subsequent exposure during war theaters pose a clinical challenge that can lead to multi-organ dysfunction and long-term complications in the elderly. Using an aged mouse model and high-throughput sequencing, this study investigated the molecular dynamics of the liver-brain connection during heat stress exposure. Distinctive gene expression patterns induced by periodic heat stress emerged in both brain and liver tissues. An altered transcriptome profile showed heat stress-induced altered acute phase response pathways, causing neural, hepatic, and systemic inflammation and impaired synaptic plasticity. Results also demonstrated that proinflammatory molecules such as S100B, IL-17, IL-33, and neurological disease signaling pathways were upregulated, while protective pathways like aryl hydrocarbon receptor signaling were downregulated. In parallel, Rantes, IRF7, NOD1/2, TREM1, and hepatic injury signaling pathways were upregulated. Furthermore, current research identified Orosomucoid 2 (ORM2) in the liver as one of the mediators of the liver-brain axis due to heat exposure. In conclusion, the transcriptome profiling in elderly heat-stressed mice revealed a coordinated network of liver-brain axis pathways with increased hepatic ORM2 secretion, possibly due to gut inflammation and dysbiosis. The above secretion of ORM2 may impact the brain through a leaky blood-brain barrier, thus emphasizing intricate multi-organ crosstalk.
Keywords: Climate change; Gut-liver-brain axis; Human health; Hyperthermia; ORM2; RANTES.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
The gut-liver axis plays a limited role in mediating the liver's heat susceptibility of Chinese giant salamander.BMC Genomics. 2025 May 13;26(1):475. doi: 10.1186/s12864-025-11644-4. BMC Genomics. 2025. PMID: 40360994 Free PMC article.
-
Liver transcriptome response to hyperthermic stress in three distinct chicken lines.BMC Genomics. 2016 Nov 22;17(1):955. doi: 10.1186/s12864-016-3291-0. BMC Genomics. 2016. PMID: 27875983 Free PMC article.
-
Hepatic transcriptome profiling according to growth rate reveals acclimation in metabolic regulatory mechanisms to cyclic heat stress in broiler chickens.Poult Sci. 2022 Dec;101(12):102167. doi: 10.1016/j.psj.2022.102167. Epub 2022 Sep 20. Poult Sci. 2022. PMID: 36257074 Free PMC article.
-
Fecal microbiota transplantation protects rotenone-induced Parkinson's disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis.Microbiome. 2021 Nov 17;9(1):226. doi: 10.1186/s40168-021-01107-9. Microbiome. 2021. PMID: 34784980 Free PMC article.
-
The Gut-Brain Axis in Multiple Sclerosis. Is Its Dysfunction a Pathological Trigger or a Consequence of the Disease?Front Immunol. 2021 Sep 21;12:718220. doi: 10.3389/fimmu.2021.718220. eCollection 2021. Front Immunol. 2021. PMID: 34621267 Free PMC article. Review.
References
-
- National Academies, Sciences, and Engineering; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. 10.17226/25837 (National Academies Press, Washington, DC, US, 2020). - PubMed
-
- Lugo-Amador, N. M., Rothenhaus, T. & Moyer, P. Heat-related illness. Emerg. Med. Clin. North Am.22, 315–327, viii (2004). 10.1016/j.emc.2004.01.004 - PubMed
-
- QuickStats: Percentage Distribution of Heat-Related Deaths,* by Age Group - National Vital Statistics System, United States, 2018–2020. MMWR Morb. Mortal Wkly. Rep.71, 808 (2022). 10.15585/mmwr.mm7124a6 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

