Discovery of a brain penetrant small molecule antagonist targeting LPA1 receptors to reduce neuroinflammation and promote remyelination in multiple sclerosis
- PMID: 38719983
- PMCID: PMC11079064
- DOI: 10.1038/s41598-024-61369-9
Discovery of a brain penetrant small molecule antagonist targeting LPA1 receptors to reduce neuroinflammation and promote remyelination in multiple sclerosis
Abstract
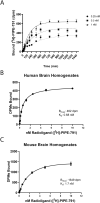
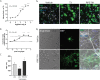
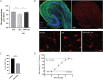
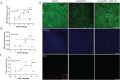
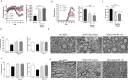
Multiple sclerosis (MS) is a chronic neurological disease characterized by inflammatory demyelination that disrupts neuronal transmission resulting in neurodegeneration progressive disability. While current treatments focus on immunosuppression to limit inflammation and further myelin loss, no approved therapies effectively promote remyelination to mitigate the progressive disability associated with chronic demyelination. Lysophosphatidic acid (LPA) is a pro-inflammatory lipid that is upregulated in MS patient plasma and cerebrospinal fluid (CSF). LPA activates the LPA1 receptor, resulting in elevated CNS cytokine and chemokine levels, infiltration of immune cells, and microglial/astrocyte activation. This results in a neuroinflammatory response leading to demyelination and suppressed remyelination. A medicinal chemistry effort identified PIPE-791, an oral, brain-penetrant, LPA1 antagonist. PIPE-791 was characterized in vitro and in vivo and was found to be a potent, selective LPA1 antagonist with slow receptor off-rate kinetics. In vitro, PIPE-791 induced OPC differentiation and promoted remyelination following a demyelinating insult. PIPE-791 further mitigated the macrophage-mediated inhibition of OPC differentiation and inhibited microglial and fibroblast activation. In vivo, the compound readily crossed the blood-brain barrier and blocked LPA1 in the CNS after oral dosing. Direct dosing of PIPE-791 in vivo increased oligodendrocyte number, and in the mouse experimental autoimmune encephalomyelitis (EAE) model of MS, we observed that PIPE-791 promoted myelination, reduced neuroinflammation, and restored visual evoked potential latencies (VEP). These findings support targeting LPA1 for remyelination and encourage development of PIPE-791 for treating MS patients with advantages not seen with current immunosuppressive disease modifying therapies.
© 2024. The Author(s).
Conflict of interest statement
All authors, while engaged in the research pertaining to this paper, were employees of Contineum Therapeutics. Contineum Therapeutics has filed patent applications related to the subject matter of this paper.
Figures
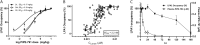


References
-
- Schmitz K, Brunkhorst R, de Bruin N, Mayer CA, Haussler A, Ferreiros N, Schiffmann S, Parnham MJ, Tunaru S, Chun J, Offermanns S, Foerch C, Scholich K, Vogt J, Wicker S, Lotsch J, Geisslinger G, Tegeder I. Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2017;5(1):42. doi: 10.1186/s40478-017-0446-4. - DOI - PMC - PubMed
-
- Lorrain, K. I., Poon, M. M., Baccei, J., Broadhead, A. Dearie, A., Green, A., Chan, J. R., Lorrain, D. L. LPA Receptors Modulate Oligodendrocyte Differentiation and Maturation (Society for Neuroscience, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

