Complete biosynthesis of QS-21 in engineered yeast
- PMID: 38720067
- PMCID: PMC11111400
- DOI: 10.1038/s41586-024-07345-9
Complete biosynthesis of QS-21 in engineered yeast
Abstract
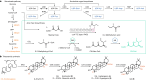
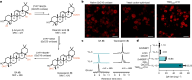
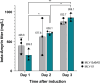
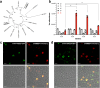
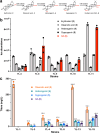
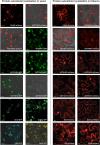
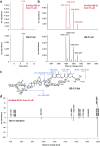
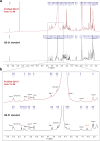
QS-21 is a potent vaccine adjuvant and remains the only saponin-based adjuvant that has been clinically approved for use in humans1,2. However, owing to the complex structure of QS-21, its availability is limited. Today, the supply depends on laborious extraction from the Chilean soapbark tree or on low-yielding total chemical synthesis3,4. Here we demonstrate the complete biosynthesis of QS-21 and its precursors, as well as structural derivatives, in engineered yeast strains. The successful biosynthesis in yeast requires fine-tuning of the host's native pathway fluxes, as well as the functional and balanced expression of 38 heterologous enzymes. The required biosynthetic pathway spans seven enzyme families-a terpene synthase, P450s, nucleotide sugar synthases, glycosyltransferases, a coenzyme A ligase, acyl transferases and polyketide synthases-from six organisms, and mimics in yeast the subcellular compartmentalization of plants from the endoplasmic reticulum membrane to the cytosol. Finally, by taking advantage of the promiscuity of certain pathway enzymes, we produced structural analogues of QS-21 using this biosynthetic platform. This microbial production scheme will allow for the future establishment of a structure-activity relationship, and will thus enable the rational design of potent vaccine adjuvants.
© 2024. The Author(s).
Conflict of interest statement
J.D.K. has a financial interest in Amyris, Demetrix, Maple Bio, Lygos, Napigen, Berkeley Yeast, Zero Acre Farms, Ansa Biotechnologies, Apertor Pharmaceuticals, ResVit Bio, and Cyklos Materials. J.R., L.B.B.M. and A.O. are inventors of patents arising from work on QS-21 pathway characterization.
Figures