Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives
- PMID: 38720342
- PMCID: PMC11077829
- DOI: 10.1186/s13045-024-01544-7
Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives
Abstract
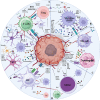
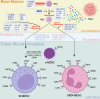
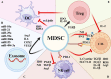
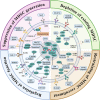
Glioblastoma (GBM), the predominant and primary malignant intracranial tumor, poses a formidable challenge due to its immunosuppressive microenvironment, thereby confounding conventional therapeutic interventions. Despite the established treatment regimen comprising surgical intervention, radiotherapy, temozolomide administration, and the exploration of emerging modalities such as immunotherapy and integration of medicine and engineering technology therapy, the efficacy of these approaches remains constrained, resulting in suboptimal prognostic outcomes. In recent years, intensive scrutiny of the inhibitory and immunosuppressive milieu within GBM has underscored the significance of cellular constituents of the GBM microenvironment and their interactions with malignant cells and neurons. Novel immune and targeted therapy strategies have emerged, offering promising avenues for advancing GBM treatment. One pivotal mechanism orchestrating immunosuppression in GBM involves the aggregation of myeloid-derived suppressor cells (MDSCs), glioma-associated macrophage/microglia (GAM), and regulatory T cells (Tregs). Among these, MDSCs, though constituting a minority (4-8%) of CD45+ cells in GBM, play a central component in fostering immune evasion and propelling tumor progression, angiogenesis, invasion, and metastasis. MDSCs deploy intricate immunosuppressive mechanisms that adapt to the dynamic tumor microenvironment (TME). Understanding the interplay between GBM and MDSCs provides a compelling basis for therapeutic interventions. This review seeks to elucidate the immune regulatory mechanisms inherent in the GBM microenvironment, explore existing therapeutic targets, and consolidate recent insights into MDSC induction and their contribution to GBM immunosuppression. Additionally, the review comprehensively surveys ongoing clinical trials and potential treatment strategies, envisioning a future where targeting MDSCs could reshape the immune landscape of GBM. Through the synergistic integration of immunotherapy with other therapeutic modalities, this approach can establish a multidisciplinary, multi-target paradigm, ultimately improving the prognosis and quality of life in patients with GBM.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Qazi MA, Vora P, Venugopal C, Sidhu SS, Moffat J, Swanton C, et al. Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol. 2017;28(7):1448–1456. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

